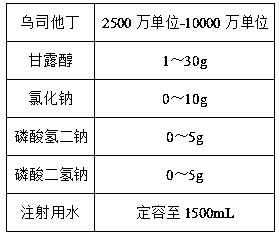
Ulinastatin injection and preparation method for same
A technology for ulinastatin and injection, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as microbial contamination, activity reduction, etc. , high stability, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
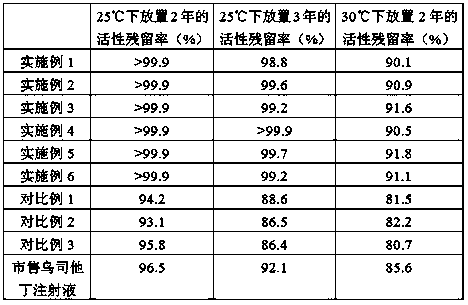
Examples
Embodiment 1
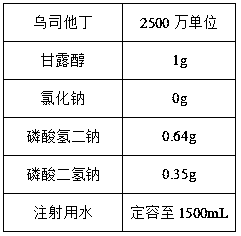
[0035] Embodiment 1: a kind of ulinastatin injection
[0036] The formula is as follows: per 1000 sticks
[0037]
[0038] Preparation method:
[0039]⑴. Excipient solution preparation: Weigh mannitol, disodium hydrogen phosphate and sodium dihydrogen phosphate according to the formula, add water for injection with a total volume of 450mL and a temperature of 2°C, stir until dissolved, add 1g and stir at 2°C for 20min for adsorption After treatment, use a 0.45 μm filter membrane to filter and decarburize to obtain an excipient solution, and keep the temperature of the solution at 2°C;
[0040] ⑵. Weigh ulinastatin according to the dosage of the formula, and add the excipient solution and ulinastatin obtained in step 1 into the liquid preparation tank in sequence, and stir until completely dissolved to obtain medicinal solution A, and keep the temperature of the medicinal solution at 2°C ;
[0041] (3) Use 3M hydrochloric acid or sodium hydroxide solution to adjust the pH...
Embodiment 2
[0045] Embodiment 2: a kind of ulinastatin injection
[0046] The prescription ratio is as follows: per 1000 sticks
[0047]
[0048] Preparation method:
[0049] ⑴. Excipient solution preparation: weigh mannitol, sodium chloride, disodium hydrogen phosphate and sodium dihydrogen phosphate according to the formula, add water for injection with a total volume of 750mL and a temperature of 3°C, stir until dissolved, add 2g at 3°C Stir under low pressure for 18 minutes after adsorption treatment, use a 0.45 μm filter membrane to filter and decarburize to obtain an excipient solution, and keep the temperature of the solution at 3°C;
[0050] ⑵. Weigh ulinastatin according to the dosage of the formula, and add the auxiliary material solution and ulinastatin obtained in step 1 into the liquid mixing tank in sequence, and stir until completely dissolved to obtain medicinal solution A, and keep the temperature of the medicinal solution at 3°C ;
[0051] (3) Use 3M hydrochloric a...
Embodiment 3
[0055] Embodiment 3: a kind of ulinastatin injection
[0056] The prescription ratio is as follows: per 1000 sticks
[0057]
[0058] Preparation method:
[0059] ⑴. Excipient solution preparation: Weigh mannitol, sodium chloride, disodium hydrogen phosphate and sodium dihydrogen phosphate according to the formula, add water for injection with a total volume of 850mL and a temperature of 3°C, stir until dissolved, add 3g at 3°C Stir under low pressure for 16 minutes after adsorption treatment, use a 0.45 μm filter membrane to filter and decarburize to obtain an excipient solution, and keep the temperature of the solution at 3°C;
[0060] ⑵. Weigh ulinastatin according to the dosage of the formula, and add the auxiliary material solution and ulinastatin obtained in step 1 into the liquid mixing tank in sequence, and stir until completely dissolved to obtain medicinal solution A, and keep the temperature of the medicinal solution at 3°C ;
[0061] (3) Use 3M hydrochloric a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com