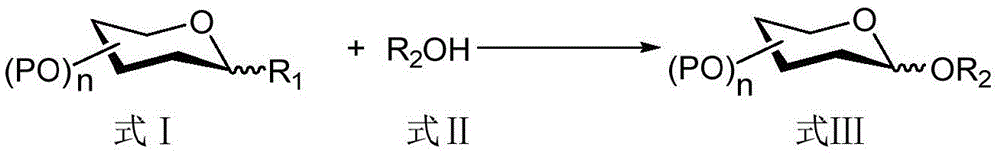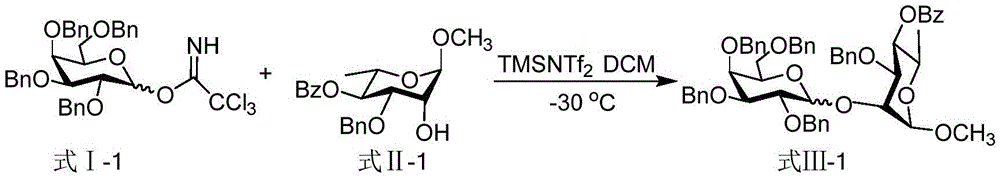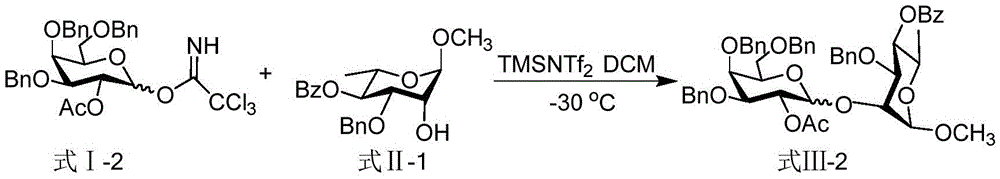Method for improving beta-glucosidic bond stereoselectivity through bis(trifluoromethane sulfonimide) reagent activation glycosylation reaction
A technology of trifluoromethanesulfonimide and glycosylation reaction, which is applied in the field of β-glycosidic bonds, can solve the problems of low stereoselectivity of glycoside formation reaction and insufficient mild reaction conditions, and achieve high stereoselectivity and reaction The effect of mild conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Taking the glycosyl coupling product shown in the synthesis formula III-1 as an example, the reaction equation is as follows:
[0014]
[0015] Dissolve 83 mg (0.12 mmol) of the glycosyl donor represented by formula I-1 and 30 mg (0.08 mmol) of the glycosyl acceptor represented by formula II-1 in 0.62 mL of dichloromethane, and stir at 0°C for 10 minutes , drop 0.18mLTMSNTf 2 (1.8 μ L, 0.008 mmol) of dichloromethane solution, continue to react for 1 hour at 0° C., quench the reaction with triethylamine, spin dry, and column separation (the volume ratio of eluent is sherwood oil and ethyl acetate is 5: 1), to obtain the glycosyl coupling product shown in formula III-1, the total yield is 94%, α:β=1.5:1, and the structural characterization data of the product are as follows:
[0016] 1 HNMR (600MHz, CDCl 3 )δ: 8.02-7.98 (m, 2H), 7.57 (t, J=7.2Hz, 1H), 7.48-7.39 (m, 5H), 7.36-7.26 (m, 11H), 7.24-7.16 (m, 6H) , 7.13-7.02(m, 5H), 5.51(t, J=9.6Hz, 0.37H), 5.42(t, J=9.6...
Embodiment 2
[0021] Taking the glycosyl coupling product shown in the synthetic formula III-2 as an example, the reaction equation is as follows:
[0022]
[0023] Dissolve 76 mg (0.12 mmol) of the glycosyl donor represented by formula I-2 and 30 mg (0.08 mmol) of the glycosyl acceptor represented by formula II-1 in 0.62 mL of dichloromethane, and stir at -30°C for 10 minutes, drop 0.18mLTMSNTf 2 (1.8 μ L, 0.008 mmol) in dichloromethane solution, continue to react at -30 ° C for 1 hour, quench the reaction with triethylamine, spin dry, and column separation (the volume ratio of petroleum ether and ethyl acetate as the eluent is 4 :1 mixed solution), obtain formula III-2 compound, its total yield is 97%, α:β=1:10.1, the structural characterization data of product are as follows:
[0024] α-configuration product: 1 HNMR (600MHz, CDCl 3)δ: 8.00-7.99 (m, 2H), 7.59 (t, J = 7.2Hz, 1H), 7.45 (t, J = 7.8Hz, 2H), 7.41 (d, J = 7.2Hz, 2H), 7.37 ( t, J=7.8Hz, 2H), 7.34-7.27(m, 6H), 7.24-7.16(m,...
Embodiment 3
[0030] Taking the glycosyl coupling product shown in the synthesis formula III-3 as an example, the reaction equation is as follows:
[0031]
[0032] Dissolve 76 mg (0.12 mmol) of the glycosyl donor represented by formula I-2 and 21 mg (0.08 mmol) of the glycosyl acceptor represented by formula II-2 in 0.62 mL of dichloromethane, and stir at -30°C for 10 minutes, drop 0.18mLTMSNTf 2 (1.8 μ L, 0.008 mmol) in dichloromethane solution, continue to react at -30 ° C for 1 hour, quench the reaction with triethylamine, spin dry, and column separation (the volume ratio of petroleum ether and ethyl acetate as the eluent is 4 :1 mixed solution), to obtain the glycosyl coupling product shown in formula III-3, its total yield is 83%, α:β=1:10, the structural characterization data of the product are as follows:
[0033] α-configuration product: 1 HNMR (600MHz, CDCl 3 )δ: 7.36-7.22 (m, 14H), 5.52 (d, J = 4.8Hz, 1H), 5.31 (dd, J = 10.2, 3.6Hz, 1H), 5.10 (d, J = 3.6Hz, 1H), 4.92(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com