Naphthalimide two-photon fluorochrome and preparation method and application thereof
A two-photon fluorescence, naphthalimide technology, applied in naphthalimide compounds and their preparation, fluorescent dyes in biological dyeing, naphthalimide two-photon fluorescent dyes, and imaging applications, can solve the problem of cytotoxicity , poor water solubility, etc., to achieve the effect of improving water solubility, good water solubility, and good transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
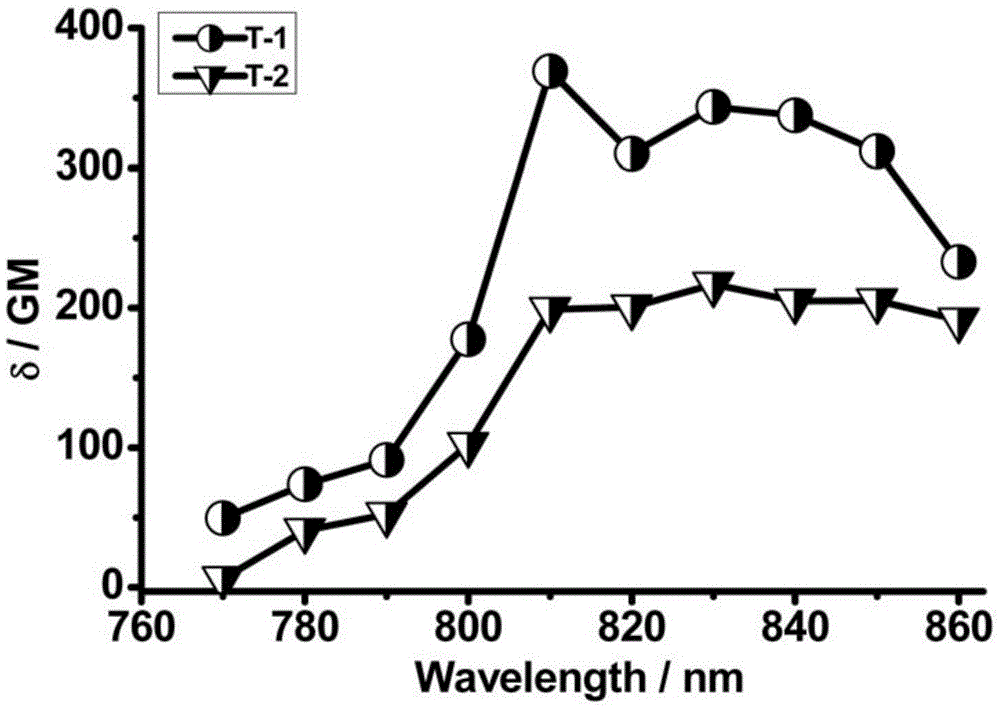
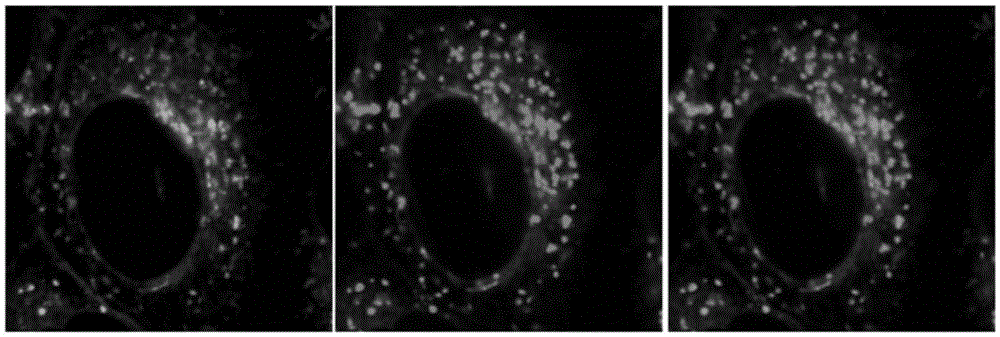
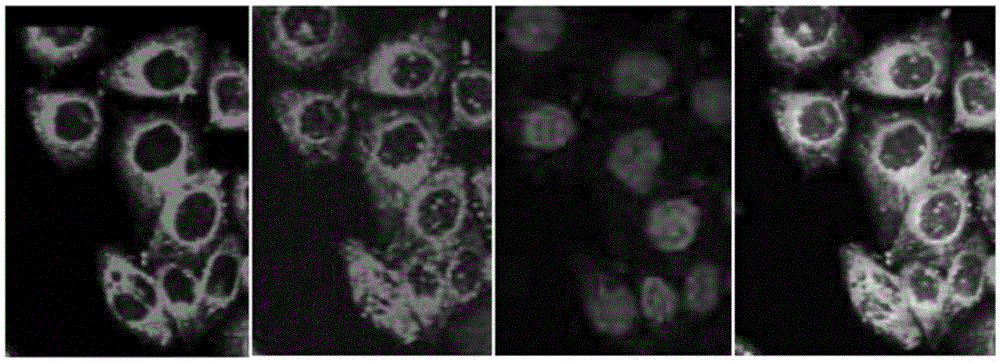
Image
Examples
Embodiment 1
[0044] Example 1: 4-branched PEI-N-butyl-1,8-naphthalimide (T-1)
[0045] The present embodiment provides a kind of naphthalimide compound 4-branched PEI-N-butyl-1,8-naphthalimide (represented by T-1 below), its structure is shown in the following formula; Wherein, PEI Be the branched PEI of molecular weight 2000;
[0046]
[0047] Its preparation method is as follows:
[0048] 1) Add 4-bromo-1,8-naphthalene anhydride (542mg, 2mmol) and n-butylamine (205μl, 2.1mmol) into ethanol (150ml) in a round-bottomed flask, heat at reflux at 80°C for 3h, and react The liquid was concentrated and precipitated, and filtered to obtain a yellow solid, which was the intermediate M-1, with a molecular weight of 331 and a yield of 90%; 1 The HNMR data are as follows:
[0049] 1 HNMR (400MHz, CDCl 3 )δ (ppm) 8.65 (dd, J = 8.0Hz and 0.8Hz, 1H), 8.56 (d, J = 8.0Hz, 1H), 8.41 (d, J = 8.0Hz, 1H), 8.03 (d, J = 8.0Hz, 1H), 7.84(t, J=8.0Hz, 1H), 4.18(t, J=8.0Hz, 2H), 1.74–1.68(m, 2H), 1.45–1.4...
Embodiment 2
[0055] Example 2: 4-branched PEI-N-(N,N-dimethylethylenediamine)-1,8-naphthalimide (T-2)
[0056] This embodiment provides a naphthalimide compound 4-branched PEI-N-(N,N-dimethylethylenediamine)-1,8-naphthalimide (hereinafter represented by T-2), Its structure is shown in the following formula; wherein, PEI is a branched PEI with a molecular weight of 2000;
[0057]
[0058] Its preparation method is as follows:
[0059] 1) Add 4-bromo-1,8-naphthalene anhydride (542mg, 2mmol) and N,N-dimethylethylenediamine (230μl, 2.1mmol) to ethanol (150ml) in a round-bottom flask, 80°C After heating to reflux for 3 hours, the reaction solution was concentrated and precipitated, and filtered to obtain a yellow solid, which was intermediate M-2, with a yield of 88%; M-2 1 The HNMR data are as follows:
[0060] 1 HNMR (400MHz, CDCl 3 )δ (ppm) 8.66 (dd, J = 7.2Hz and 1.2Hz, 1H), 8.57 (dd, J = 8.0Hz and 0.8Hz, 1H), 8.41 (d, J = 8.0Hz, 1H), 8.04 (d ,J=8.0Hz,1H),7.84(t,J=8.0Hz,1H),4.32(t,...
Embodiment 3
[0064] Example 3: 4-branched PEI-N-n-hexadecylamino-1,8-naphthalimide (T-3)
[0065] This embodiment provides a naphthalimide compound 4-branched PEI-N-n-hexadecylamino-1,8-naphthalimide (hereinafter represented by T-3), its structure is shown in the following formula; Wherein, PEI is the branched PEI of molecular weight 2000;
[0066]
[0067] Its preparation method is as follows:
[0068] 1) Add 4-bromo-1,8-naphthalene anhydride (542mg, 2mmol) and n-hexadecylamine (507mg, 2.1mmol) into ethanol (150ml) in a round bottom flask, heat at reflux at 80°C for 3h, The reaction solution was concentrated and precipitated, and filtered to obtain a yellow solid, which was intermediate M-3, with a yield of 85%; M-3 1 The HNMR data are as follows:
[0069] 1 HNMR (400MHz, CDCl 3 )δ (ppm) 8.67 (d, J = 6.4Hz, 1H), 8.57 (d, J = 8.4Hz, 1H), 8.41 (d, J = 7.6Hz, 1H), 8.04 (d, J = 8.0Hz, 1H), 7.84(t, J=7.6Hz, 1H), 4.16(t, J=7.6Hz, 2H), 1.76–1.26(m, 28H), 0.88(t, J=6.4Hz, 3H).
[0070] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com