Dibenzo quinolizine compound entity and application thereof
A technology of dibenzoquinazine and benzoquinazine, which is applied in the field of dibenzoquinazine compound entities and their uses, to achieve the effects of improving hygroscopicity, facilitating storage and transportation, and improving operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
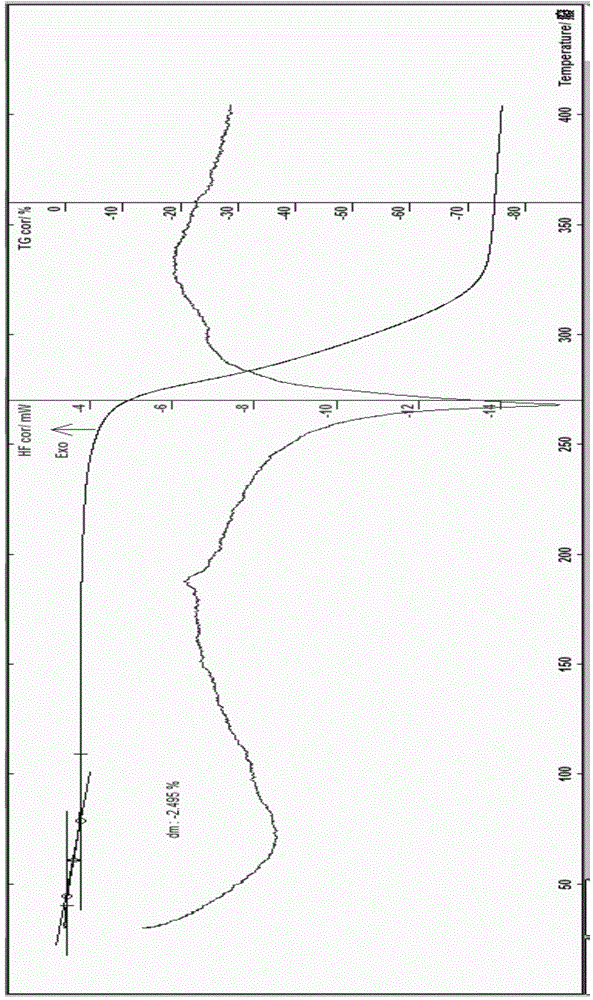
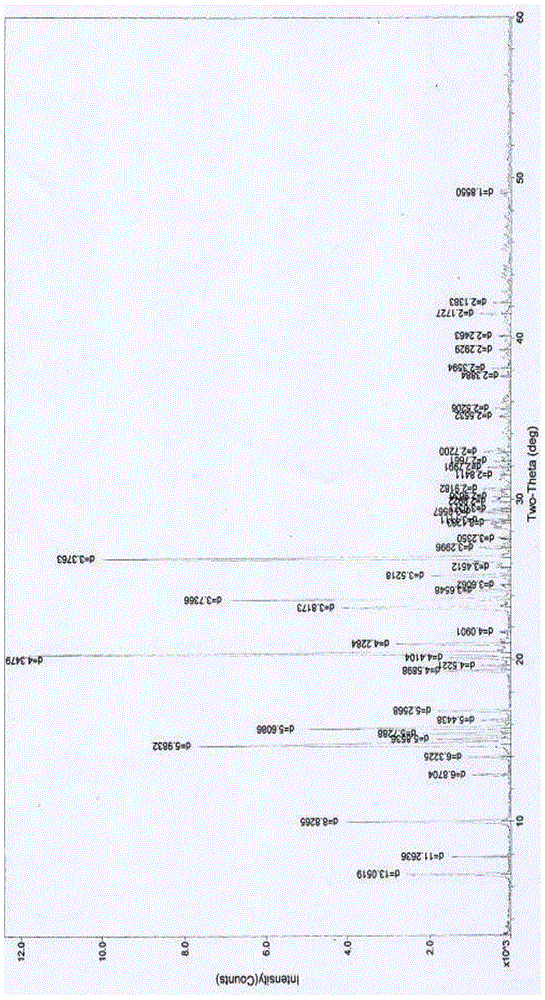
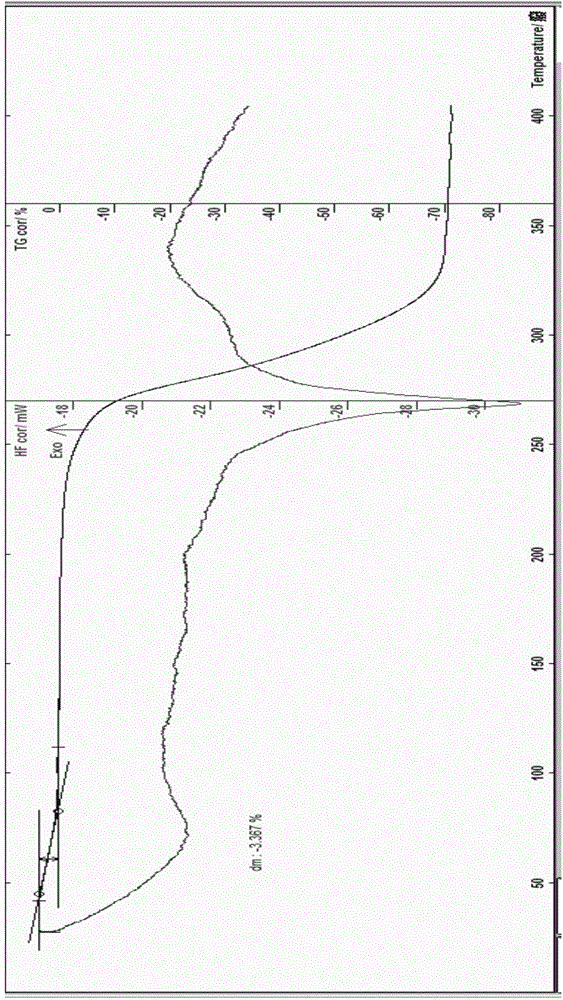
[0067] The preparation of embodiment 1 rotundine hydrochloride 0.5 hydrate gets the fine powder 6.22g of rotundine in 250ml flask, adds 50ml of water, stirs, heats, adds the hydrochloric acid solution of appropriate amount 4M, stirs, control solution pH=below 1.5, Continue to stir for about 4 hours, filter, decompress and concentrate for a while, place, slowly cool to about 0°C, place, wait for the precipitate to be fully separated, filter, and wash the solid obtained three times with ice-cold water and a small amount of ice-cold ethanol, and filter with suction , dilute the obtained crystals, blow dry at about 25°C for 3 hours, and then dry at about 50°C for about 5 hours to obtain 5.53 grams of off-white solid; Identification: 1) Take 10 mg of the product prepared in this example, add 2ml of water to dissolve it , add 1 drop of potassium dichromate test solution, promptly produce yellow precipitate; (2) get the product 10mg that this embodiment prepares, add 2ml water to diss...
Embodiment 2
[0068] The preparation of embodiment 2 rotundine hydrochloride 0.5 hydrate gets the fine powder 5.36g of rotundine, ethanol 5ml, water 50ml in the 250ml flask, stirs, heats, feeds hydrogen chloride gas, stirs, control solution pH=below 1.5, Continue to stir for about 4 hours, slowly cool to about -5°C, place it until the precipitate is fully separated, filter, wash with ice-cold ethanol, filter with suction, dilute the obtained crystals, and dry it with air at about 28°C for 6 hours, then re-dry it at about 40°C Dry it in a vacuum oven for about 2 hours (the degree of vacuum is about 0.08 MPa), and obtain 2.47 grams of off-white solid; Identification: 1) Take 10 mg of the product prepared in this example, add 2 ml of water to dissolve, add 1 drop of potassium dichromate test solution , that is, a yellow precipitate is produced; (2) take 10 mg of the product prepared in this embodiment, add 2 ml of water to dissolve, add 1 drop of dilute potassium ferricyanide test solution, tha...
Embodiment 3
[0069] The preparation of embodiment 3 rotundine hydrochloride 0.75 hydrate gets the fine powder 5.36g of rotundine, adds ethanol 50ml in the 250ml flask, stirs, and heating makes dissolving, adds the hydrochloric acid solution of right amount 6M, stirs, and controls solution pH= Below 1.5, stir for about 30 minutes, decompress and concentrate for a while, let stand, slowly cool to about -20°C, let stand overnight, wait until the precipitate is fully separated, filter, wash with ice-cold ethanol, and suction filter, put the obtained solid in a flask , add 2ml of water, add an appropriate volume of 90% ethanol and heat it at about 78°C to dissolve, carry out recrystallization operation, keep it below 0°C, place it until the crystals are fully separated, filter with suction, dilute the obtained crystals, and drum at about 28°C Air-dried for 3 hours, then dried at about 60°C for about 2 hours to obtain 3.23 grams of light yellow crystals; identification: 1) Take 10 mg of the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com