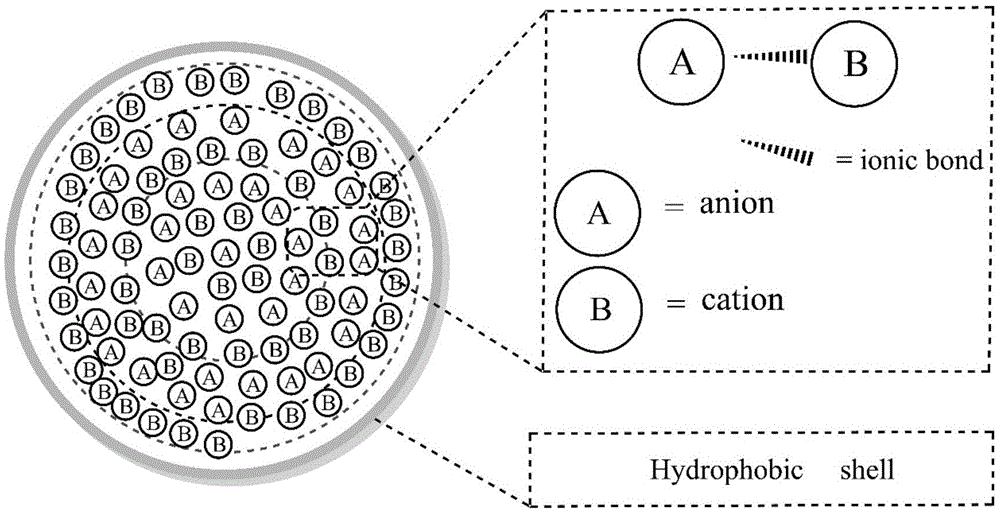
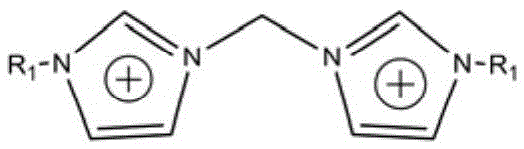
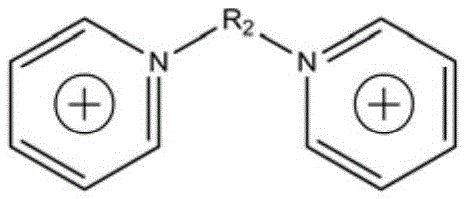
Supermolecular flame retardant based on ionic bonds and preparation method thereof
An ionic bond and supramolecular technology, applied in the field of new structural flame retardants, can solve the problems of not well-solved flame retardant efficiency and limited improvement, and achieve easy implementation, increase compatibility, and improve heat resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Take 3.5g (0.0103mol) of polyphosphoric acid and disperse it in 80ml of absolute ethanol, heat it in an oil bath at 70°C and stir under reflux, and blow nitrogen into it to prepare a solution E with a dispersion concentration of 0.1287mol / L. Dissolve 9g (0.0832mol) of p-phenylenediamine in 150ml of absolute ethanol, adjust the pH value to about 6.5 with formic acid, and dissolve it under heating at 60°C to prepare an acidified concentration of 0.5547mol / L p-phenylenediamine Solution D. Take 50ml of solution D and add it dropwise to E, and drop it into the flask at a rate of 1-2 drops per second to form a precipitate. After a reaction time of 3 hours, add 2g of octadecylamine (0.0074mol) to it. After reflux for 2 hours, remove the solvent by filtration, wash with ethanol three times, dry the product in a blast oven at 70°C for 1 hour, transfer to a vacuum oven at 50°C for 5 hours, and grind it into powder to obtain the product after drying. The resulting structure is as...
Embodiment 2
[0060] Take the dicationic imidazolium chloride salt: Dissolve in absolute ethanol solution, fully dissolve it under heating conditions and prepare a 1.25mol / L solution for later use. Take 100ml of absolute ethanol in a 500ml three-necked flask, and add 3.8g (0.0112mol) of polyphosphoric acid into it, and mechanically stir for 1 hour under the condition of heating an oil bath at 70°C. At the same time, nitrogen protection is introduced and an exhaust gas treatment device is connected after the condenser. Take 25ml (0.0313mol) of the prepared imidazolium chloride salt solution in a constant pressure dropping funnel, and drop it into the flask at a rate of 1-2 drops per second. After a reaction time of 2 h, 2 g (0.0074 mol) of octadecylamine was added thereto. After refluxing for 2 hours, remove the solvent by filtration, wash with ethanol three times, dry the product in a blast oven at 80°C for 1 hour, transfer to a vacuum oven at 60°C for 5 hours, and grind it into powder t...
Embodiment 3
[0062] Take the dicationic imidazolium chloride salt: Dissolve in absolute ethanol solution, fully dissolve it under heating conditions and prepare a 1.25mol / L solution for later use. Take 100ml of absolute ethanol in a 500ml three-neck flask, and add 5g (0.0148mol) of polyphosphoric acid into it, and heat it in an oil bath at 70°C for 1 hour under mechanical stirring. At the same time, nitrogen protection is introduced and an exhaust gas treatment device is connected after the condenser. Take 30ml (0.0375mol) of the prepared imidazolium chloride salt solution in a constant pressure dropping funnel, drop it into the flask at a rate of 1-2 drops per second, and add 4g (0.018mol) KH550 into it after a reaction time of 2 hours. After refluxing for 2 hours, remove the solvent by filtration, wash with ethanol three times, dry the product in a blast oven at 80°C for 1 hour, transfer to a vacuum oven at 60°C for 5 hours, and grind it into powder to obtain the product after drying. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com