Non-classical secretory protein and application thereof to protein secretory expression
A technology for protein secretion and protein secretion, which is applied in the field of genetic engineering and can solve problems such as cytoplasmic protein leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
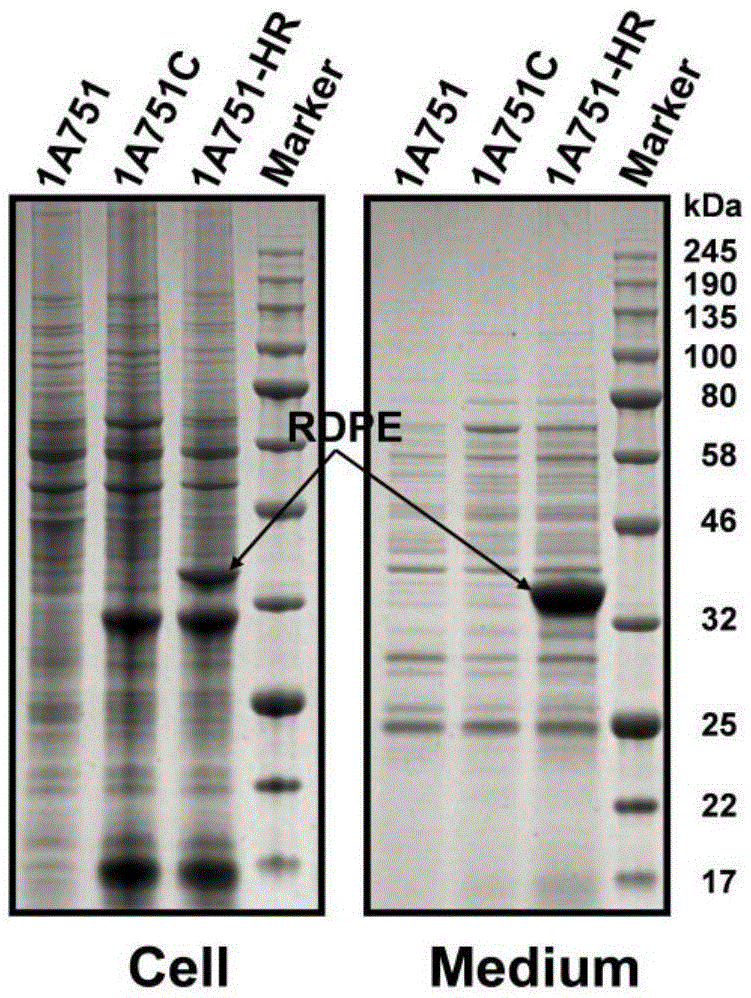
[0034] The secretory expression of embodiment 1RDPE in Bacillus subtilis
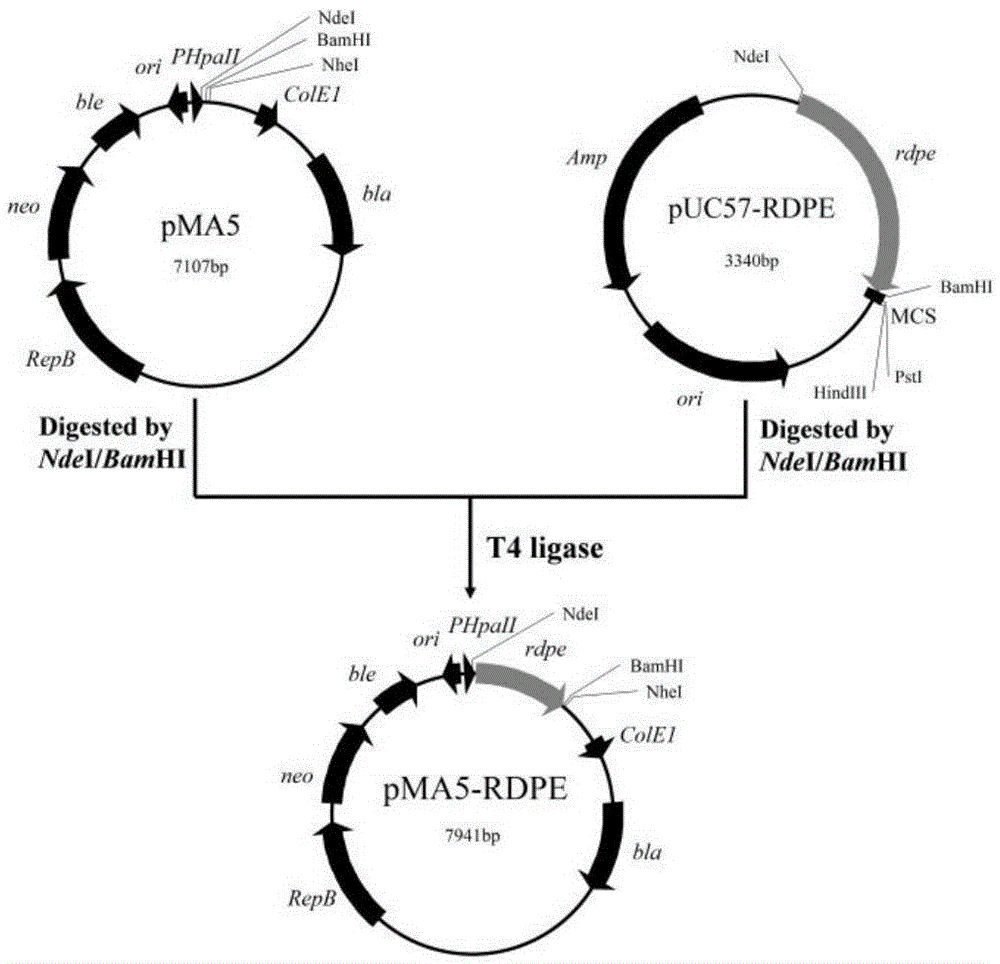
[0035] According to the sequence of the DPEase coding gene rdpe derived from Ruminococcussp. Subsequent expression vector construction. The synthesized gene was connected to the pUC57 vector and named pUC57-RDPE.
[0036] Plasmids pUC57-RDPE and pMA5 were subjected to NdeI and BamHI double enzyme digestion treatment respectively; the double enzyme digestion reaction conditions were 37°C water bath for 3h. The double-digested products were recovered by gel respectively, and the rdpe fragment with NdeI and BamHI restriction sites at both ends and the linearized pMA5 vector were obtained, and the two were ligated with T4 ligase at room temperature for 30 minutes; the ligated product was transformed Colony competent cells DH5α were screened for resistance with ampicillin (100 μg / mL), positive clones were screened by colony PCR, and plasmids were extracted for sequencing verification. For plasmid construc...
Embodiment 2
[0039] Example 2 SignalP predicts the signal peptide or signal sequence of RDPE
[0040] Using the signal peptide prediction software SignalP4.1 (http: / / www.cbs.dtu.dk / services / SignalP / ) to analyze the amino acid sequence of RDPE, it was found that there was no typical signal peptide at the N-terminus of RDPE, and there were no typical signal peptides in other regions. No secretion signal sequence is present.
Embodiment 3
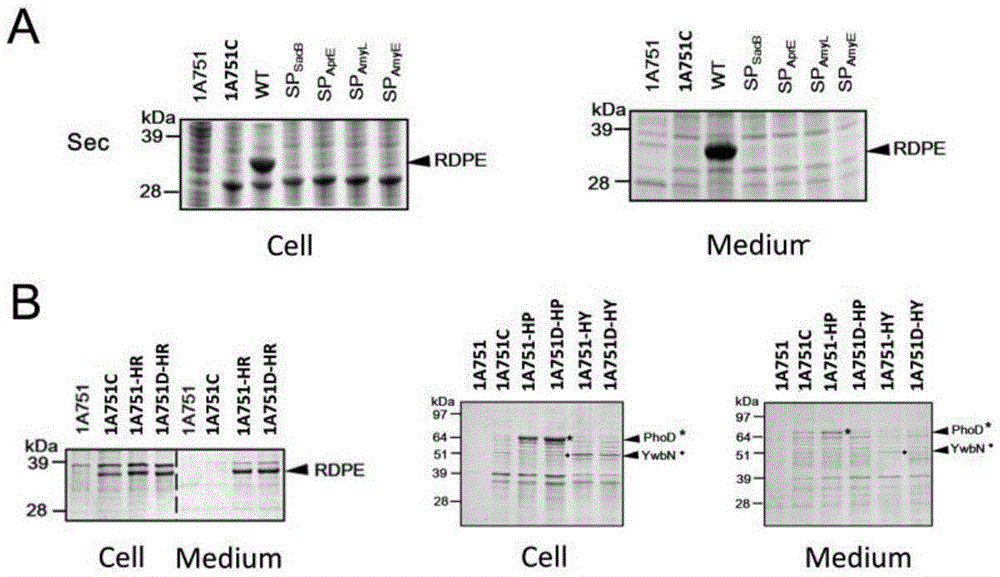
[0041] Example 3RDPE cannot be secreted by Sec and Tat secretion pathways
[0042] The construction method of this partial recombinant plasmid is based on the method in the literature (You et al. ApplEnvironMicrobiol2012, 78:1593-5). The four Sec signal peptides (SP sacB 、SP AprE 、SP AmyL and SP AmyE , the nucleic acid sequences are respectively shown in SEQ ID NO.2, 3, 4 and 5), and the PCR products obtained by cloning were respectively gel-recovered. Using pMA5-RDPE as a template, primers were used to amplify the linearized vector pMA5-RDPE, and the resulting PCR product was gel-recovered. The above four signal peptide fragments and the linearized vector pMA5-RDPE were subjected to POE-PCR (ProlongedoverlapextensionPCR), and the obtained fusion products were directly transformed into colon competent cells DH5α, respectively, to obtain recombinant expression plasmids pMA5-SP sacB -RDPE, pMA5-SP AprE -RDPE, pMA5-SP AmyL -RDPE and pMA5-SP AmyE -RDPE. The constructed pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com