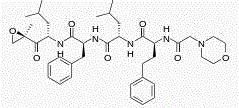
Stable carfilzomib pharmaceutical composition
A technology of carfilzomib and composition, which is applied in the direction of drug combination, antineoplastic drugs, and pharmaceutical formulations, and can solve problems such as low dissolution rate and poor fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
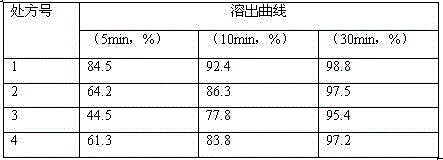
Embodiment 1
[0034] The formula composition of carfilzomib made into 1000 tablets is as follows:
[0035] Carfilzomib 30g
[0036] Croscarmellose Sodium 50g
[0037] Microcrystalline cellulose 70g
[0038] Micropowder silica gel 0.5g
[0039] Appropriate amount of 10% pregelatinized starch solution
[0040] Preparation
[0041] 1) Carfilzomib, croscarmellose sodium and microcrystalline cellulose were dried, pulverized, and passed through a 100-mesh sieve to obtain carfilzomib powder, croscarmellose sodium and microcrystalline cellulose respectively. Cellulose powder;
[0042] 2) Take the prescribed amount of carfilzomib, croscarmellose sodium and microcrystalline cellulose, mix well, then add 10% pregelatinized starch solution in an appropriate amount to make a soft material, pass through a 40-mesh sieve to granulate , to obtain carfilzomib wet granules;
[0043] 3) Dry the above-mentioned carfilzomib wet granules at 60°C until the water content is less than 5%, to obtain carfilzomi...
Embodiment 2
[0046] The formula composition of carfilzomib made into 1000 tablets is as follows:
[0047] Carfilzomib 60g
[0048] Croscarmellose Sodium 100g
[0049] Microcrystalline cellulose 140g
[0050] Micropowder silica gel 1g
[0051] Appropriate amount of 10% pregelatinized starch solution
[0052] Preparation
[0053] 1) Carfilzomib, croscarmellose sodium and microcrystalline cellulose were dried, pulverized, and passed through a 100-mesh sieve to obtain carfilzomib powder and microcrystalline cellulose powder respectively;
[0054] 2) Take the prescribed amount of carfilzomib, croscarmellose sodium and microcrystalline cellulose, mix well, then add 10% pregelatinized starch solution in an appropriate amount to make a soft material, pass through a 40-mesh sieve to granulate , to obtain carfilzomib wet granules;
[0055] 3) drying the above-mentioned carfilzomib wet granules at 60°C until the water content is less than 5%, to obtain carfilzomib dry granules;
[0056] 4) Pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com