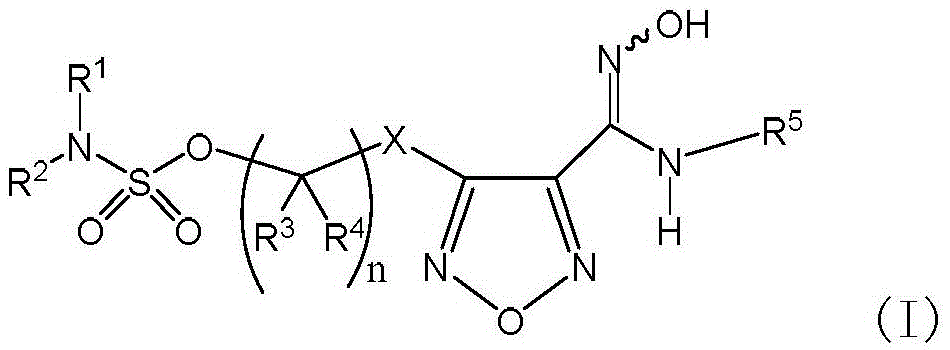
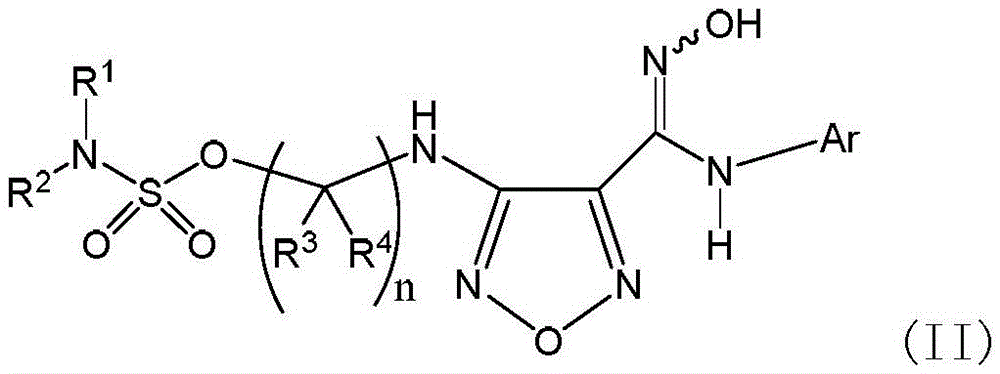
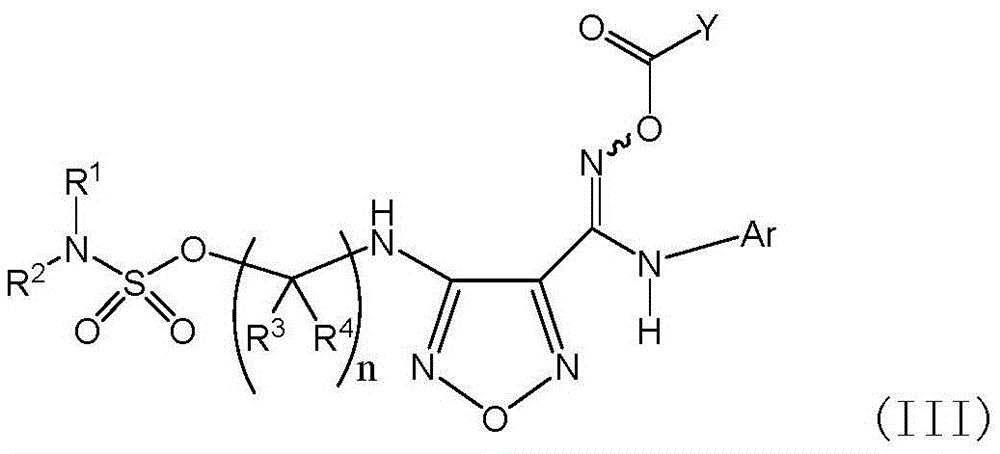
Sulfamate serving as indoleamine-2, 3-dioxygenase inhibitor and preparation method and application thereof
A C3-C10, C6-C20 technology applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] (Z)-2-((4-(nitrogen-(3-bromo-4-fluorophenyl)-N'-hydroxyimine-1,2,5-oxadiazole-3)amino)ethoxy Sulfonamide;
[0203]
[0204] The first step: the preparation of 4-amino-nitrogen'-hydroxyl-1,2,5-oxadiazole-3-carboxamidine (compound D)
[0205]
[0206] Dissolve malondicyanide (32.0g, 485mmol) in 600mL water and heat until completely dissolved. Under cooling in an ice-water bath, sodium nitrite (38.0 g, 533 mmol) and 6N hydrochloric acid (5.5 mL) were added. After reacting in an ice bath for 0.5 hours, the temperature was raised to room temperature and reacted for 2 hours. The reaction solution was continued to be cooled in an ice bath, and 50% aqueous solution of hydroxylamine hydrochloride (99.0 g, 1500 mmol) was added dropwise to the reaction solution. After stirring for half an hour, it was raised to room temperature for 1 hour of reaction. Heat to reflux for 2 hours. After the reaction is complete, adjust the pH to 7.0 with 80 mL of 6N hydrochloric acid in an ...
Embodiment 2
[0248] (Z)-2-((4-(nitrogen-(3-bromo-4-fluorophenyl)-N'-hydroxyimine-1,2,5-oxadiazole-3)amino)ethoxy Methylsulfonamide;
[0249]
[0250] According to the preparation method of Example 1, compound K (50mg, 0.13mmol) was dissolved in 1mLDMA, methylaminosulfonyl chloride (34mg, 0.26mmol) was added dropwise under ice cooling, and reacted at room temperature for 3 hours, and then the reaction mixture was poured into 3mL of water , the precipitated solid was collected by filtration to obtain 60 mg of an intermediate as a white solid, and then the intermediate was treated under the same conditions as in the tenth step of Example 1 to obtain 20 mg of Example 2 as a white solid.
[0251] 1 HNMR (400MHz, CDCl 3 ):δ7.46(s,1H),7.24(dd,1H),7.07(t,1H),6.95(m,1H),6.92(s,1H),6.15(t,1H),4.53(d, 1H), 4.38(t, 2H), 3.71(q, 3H), 2.81(d, 3H);
[0252] MSESI: m / z=452.6, [M+H] + .
Embodiment 3
[0254] (Z)-2-((4-(nitrogen-(3-bromo-4-fluorophenyl)-N'-hydroxyimine-1,2,5-oxadiazole-3)amino)ethoxy Ethylsulfonamide;
[0255]
[0256] According to the preparation method of Example 2, compound K (50 mg, 0.13 mmol) was reacted with ethylsulfonyl chloride, and then hydrolyzed to obtain Example 3 as a white solid.
[0257] 1 HNMR (400MHz, CDCl 3):δ7.55(s,1H),7.26(dd,1H),7.03(t,1H),6.93(m,1H),6.92(s,1H),6.14(t,1H),4.58(t, 1H), 4.38(t,2H), 3.72(m,2H), 3.21(m,2H), 1.20(t,3H);
[0258] MSESI: m / z=466.6, [M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com