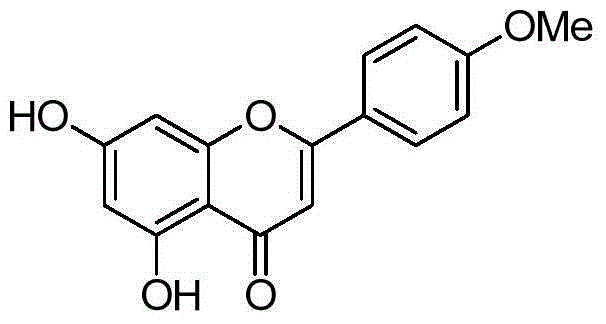
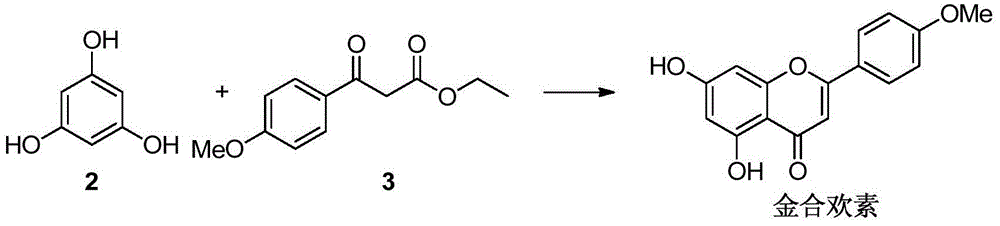
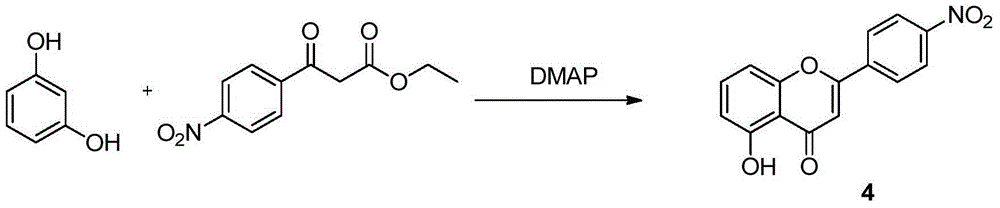
A preparing method of acacetin
The technology of acacetin and compound is applied in the field of preparation of acacetin, which can solve the problems of cumbersome operation, high cost and low yield, and achieve the effect of simple operation, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Under the conditions of anhydrous, oxygen-free, and nitrogen protection, compound 2 (1.1g) and compound 3 (3.1g) were placed in a 50ml reaction flask, 4-dimethylaminopyridine (53mg) was added, and at a temperature of 200°C The reaction was carried out for 1.5 hours. After cooling, a solid precipitated out. Suction filtration and column chromatography gave 2.2 g of acacetin, with a yield of 89%, HPLC purity of 99.35%, and mp: 266°C.
Embodiment 2
[0026] Under the conditions of anhydrous, oxygen-free, and nitrogen protection, compound 2 (1.1g) and compound 3 (3.9g) were placed in a 50ml reaction flask, 4-pyrrolidinylpyridine (130mg) was added, and at a temperature of 180°C, The reaction was carried out for 3 hours. After cooling, a solid precipitated out. Suction filtration and column chromatography gave 2.1 g of acacetin, with a yield of 86%, HPLC purity of 97.8%, and mp: 264°C.
Embodiment 3
[0028] Under the conditions of anhydrous, oxygen-free, and nitrogen protection, compound 2 (1.1g) and compound 3 (1.9g) were placed in a 50ml reaction flask, tri-n-butylphosphine (133mg) was added, and at a temperature of 190°C, The reaction was refluxed for 2 hours. After cooling, a solid precipitated out. Suction filtration and column chromatography gave 1.86 g of acacetin with a yield of 75%. HPLC purity was 99.5%, and mp: 267°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com