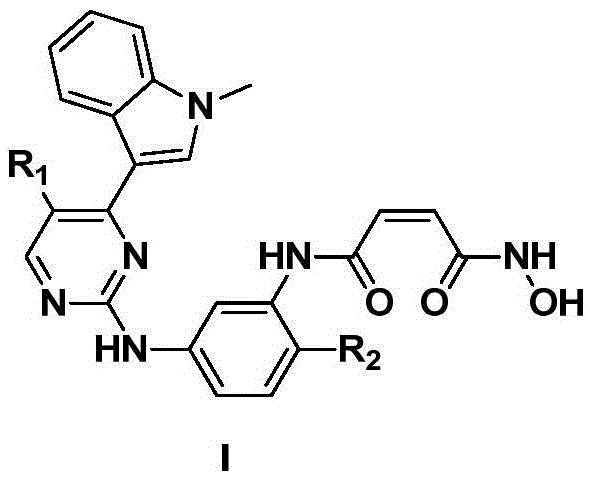
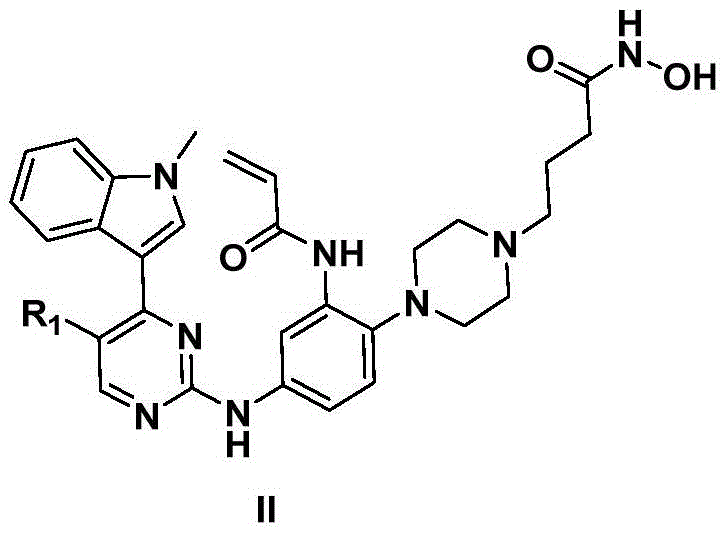
2-arylamine pyrimidine derivatives containing hydroxamic acid fragments and preparation and application
A technology of arylaminopyrimidine and hydroxamic acid, which is applied in the field of 2-arylaminopyrimidine derivatives, can solve the problems of poor selectivity of proliferation inhibition and adverse drug reactions, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1N
[0035] Example 1N 1 -2-fluoro-5-((4-(1-methyl-1H-indol-3-yl)-pyrimidin-2-yl)amino)phenyl)-N 4 -Hydroxymaleamide hydrochloride (compound 1)
[0036]
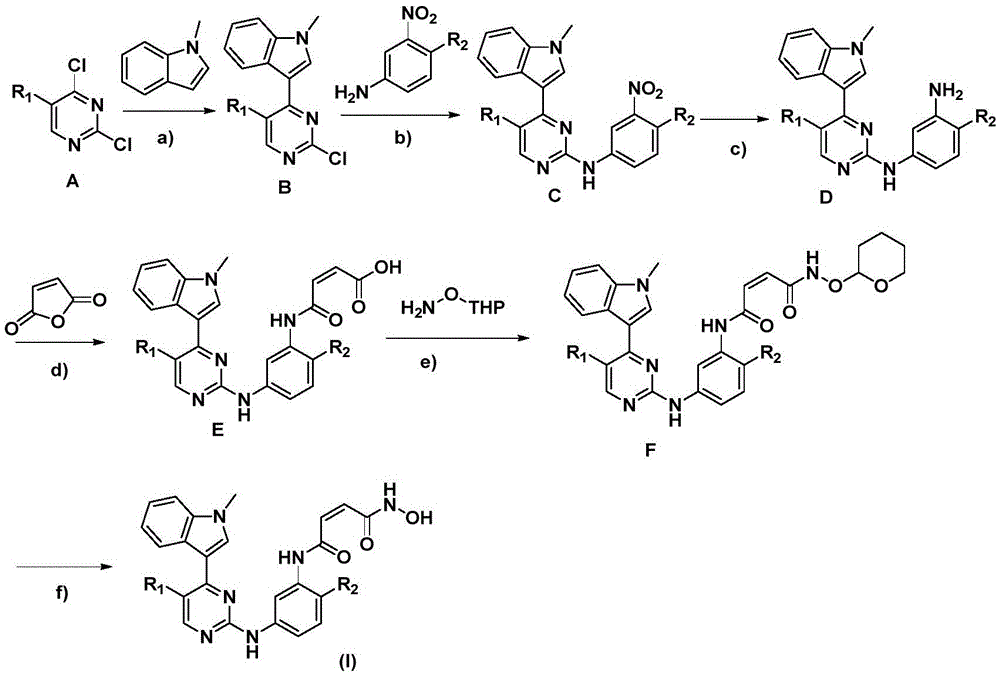
[0037] Reagents and reaction conditions: 1) dichloromethane, room temperature, 3-5 hours; 2) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1-hydroxybenzotri Azole, dichloromethane:N,N-dimethylformamide=2:1, 45°C, 5 hours; 3) 1M hydrochloric acid ether solution, 30 minutes.
[0038] Step 1: (Z)-4-((2-fluoro-5-((4-(N-methyl-3-indolyl)-2-pyrimidine)amino)phenyl)amino)-4-oxo - Preparation of 2-butenoic acid Raw material 1: 2-(4-fluoro-3-aminophenyl)-4-(N-methylindole)-2-aminopyrimidine According to J.Med.Chem.2014,57 , prepared by the method of 8249-8267.
[0039] Dissolve 2-(4-fluoro-3-aminophenyl)-4-(N-methylindole)-2-aminopyrimidine (1mmol) and maleic anhydride (1.2mmol) in 15mL dichloroethane , react for 3-5 hours. After the reaction, spin dry under reduced pressure. Recrystallization from acetone gives a...
Embodiment 2
[0048] Example 2N 1 -5-((4-(1-methyl-1H-indol-3-yl)-pyrimidin-2-yl)amino)phenyl)-N 4 -Hydroxymaleamide hydrochloride
[0049] Its structural formula is:
[0050]
[0051] Yellowsolid; m.p.: 181.0-181.4℃; 1HNMR(500MHz,DMSO-d6)δ11.73(s,1H),10.98(s,1H),10.36(s,1H),8.85(s,1H),8.30(d,J=6.6Hz,2H), 8.07(s,1H),7.61(d,J=8.3Hz,1H),7.51(d,J=8.0Hz,1H),7.48–7.45(m,2H),7.39–7.23(m,3H),7.17 (t, J=7.4Hz, 1H), 6.38(d, J=12.1Hz, 1H), 6.24(d, J=12.1Hz, 1H), 3.93(s, 3H).
[0052] 13 CNMR (125MHz, DMSO-d6) δ169.1, 167.0, 158.1, 157.6, 157.3, 156.4, 137.1, 136.6, 135.3, 135.2, 131.9, 130.0, 126.5, 123.3, 122.7, 121.2, 116.0, 115.4, 100.8, 115 33.2. HRMS(ESI)calcd.forC 23 h 21 N 6 o 3 [M+H] + = 429.1670, found 429.1670.
Embodiment 3
[0053] Example 3N 1 -(2-(Dimethylamino)-5-((4-(1-methyl-1H-indol-3-yl)-pyrimidin-2-yl)amino)phenyl)-N 4 -Hydroxymaleamide hydrochloride
[0054] Its structural formula is:
[0055]
[0056] Yellowsolid; m.p.: 217.9–218.3°C; 1 HNMR(500MHz,DMSO-d6)δ10.31(s,3H),7.65(d,J=7.8Hz,1H),7.47–7.39(m,6H),7.24–7.18(m,3H),6.99(s ,1H),6.78(d,J=12.1Hz,1H),6.28(d,J=12.1Hz,1H),3.81(s,3H),3.80(s,1H),2.08–2.01(m,6H) ,1.91(s,1H).HRMS(ESI)calcd.forC 25 h 26 N 7 o 3 [M+H] + = 472.2092, found 472.2095.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com