Uses of 2-hydroxy-N-(4-hydroxyphenyl)-benzamide compounds in preparation of tyrosinase inhibitors
A compound and composition technology, applied in the field of medicine and food, can solve the problems of long-term safety and unsatisfactory tyrosinase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0098] Example 14-Hydroxy salicylanilide compound is tested for the impact on the viability of B16 cells (C57BL / 6 mouse melanoma cells)
[0099] 1.1 Experimental method
[0100] Using MTT method: B16 cells were seeded in 96-well plates, 2×10 3 Each well, 100 μL / well, set up quadruple wells, add an equal volume of culture medium (high glucose DMEM containing 10% fetal bovine serum FBS) to zero wells (no cells, only containing the same amount of medium as the experimental wells) . After 24 hours, different concentrations (3.125 μM, 6.25 μM, 12.5 μM, 25 μM, 50 μM, 100 μM) of 4-hydroxysalicylanilide (prepared in culture medium) were added, and equal concentrations of DMSO (0.1%) solution (culture medium) were added to control wells. preparation). After 48 hours of administration, discard the medium, add 100 μL MTT solution (0.5 mg / mL, prepared in DMEM), discard the supernatant after 4 hours, add 100 μL DMSO, shake for 5 minutes to completely dissolve the crystals, and measure t...
Embodiment 24
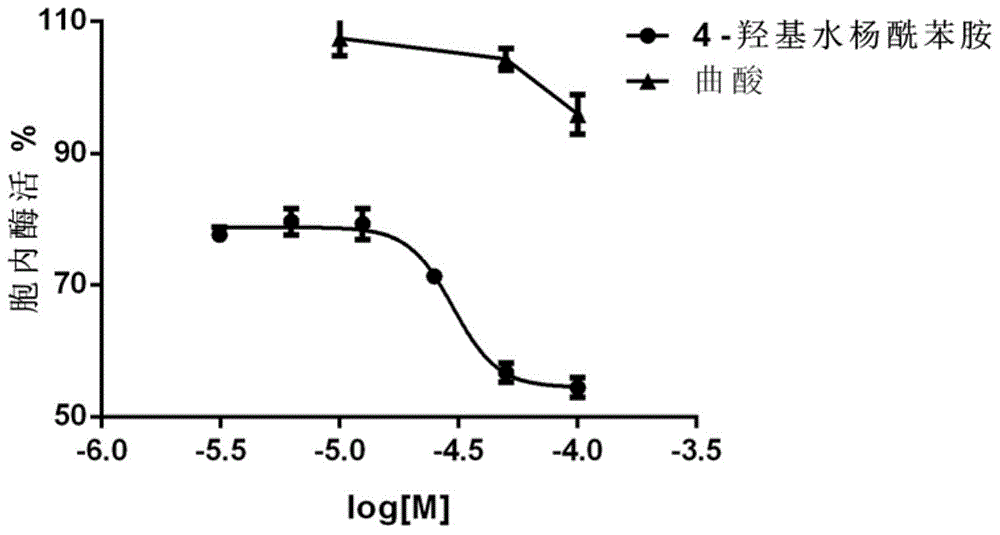
[0109] Example 2 Test of the influence of 4-hydroxy salicylanilide compound on intracellular tyrosinase activity in B16 cells
[0110] 2.1 Experimental method
[0111] B16 cells were seeded in 96-well plates, 2×10 3 Each well was cultured in high-glucose DMEM containing 10% fetal bovine serum FBS. After 24 hours, different concentrations (3.125 μM, 6.25 μM, 12.5 μM, 25 μM) of 4-hydroxysalicylanilide solution (prepared in culture medium containing 200 μM IBMX) were added. Discard the medium after 72 hours, add 100 μL of PBS solution containing 2 mM of the substrate levodopa, TritonX-1000.2%, react at 37 °C for 60 min, and measure OD with a microplate reader MultiskanMK3 492 .
[0112] 2.2 Experimental results
[0113] Table 2 Inhibitory activity to intracellular tyrosinase
[0114] compound
IC 50 (μM)
>100
4-Hydroxysalicylanilide
30.34±5.30
[0115] The results are shown in Table 2 and figure 1 As shown, kojic acid did...
Embodiment 34
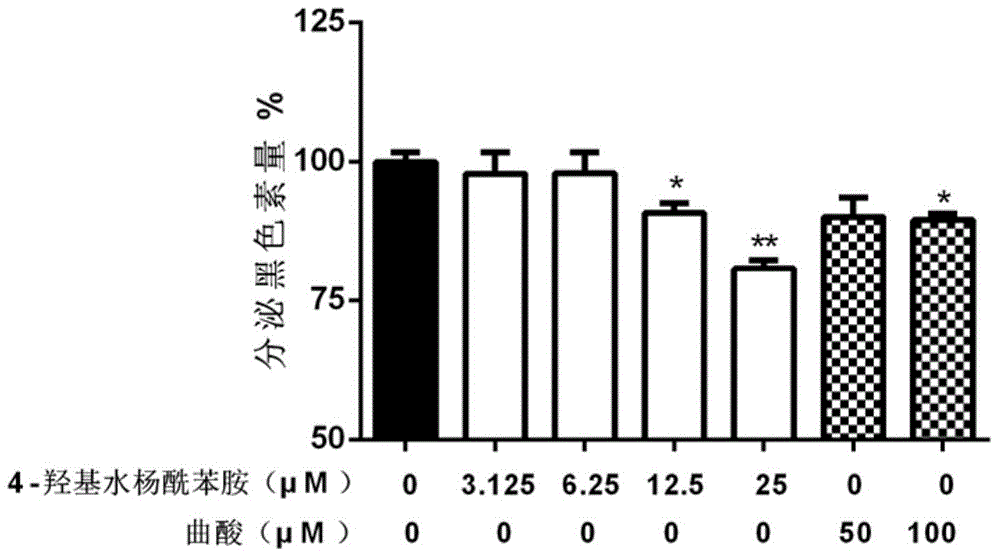
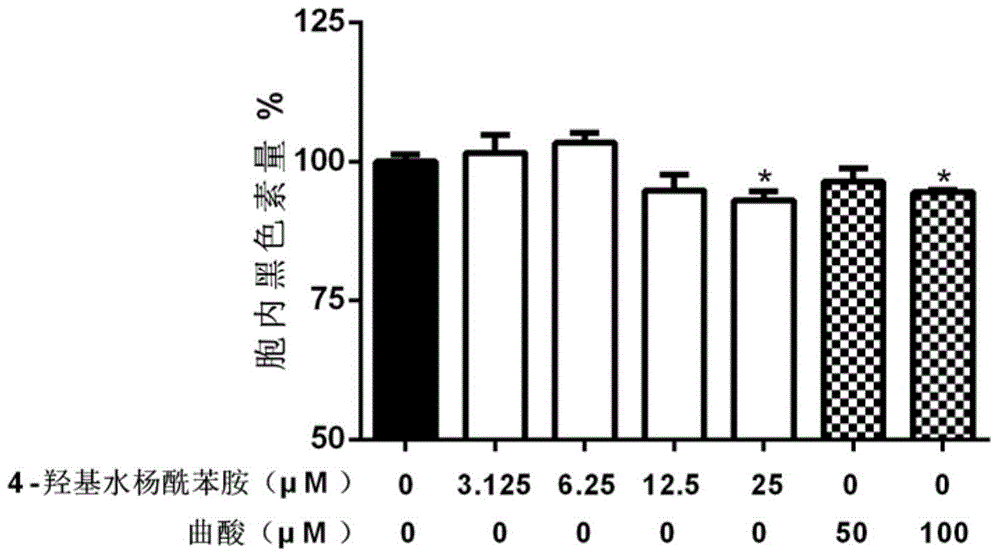
[0118] Example 3 Effect test of 4-hydroxy salicylanilide compound on melanin production in B16 cells
[0119] 3.1 Experimental method
[0120] B16 cells were seeded in 24-well plates, 2×10 4 cells / well, 1 mL / well, cultured with high-glucose DMEM containing 10% fetal bovine serum FBS. After 24 hours, 1 mL / well of medium containing 3-isobutyl-1-methylxanthine (IBMX, 200 μM) was added, and 4-hydroxyl Salicylanilide solution, using kojic acid as a positive control. After 72 hours of action, draw 100 μL of medium, and use a microplate reader MultiskanMK3 to measure OD 492 , measure the content of secreted melanin; discard the medium, wash twice with PBS, add 100 μL NaOH (1M) solution to each well, bathe in 70°C water for 20 minutes, draw the solution into a 96-well plate, and measure OD with a microplate reader MultiskanMK3 492 , Determination of intracellular melanin content.
[0121] 3.2 Experimental results
[0122] The result is as figure 2 , image 3 , Table 3 and Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com