Human sDR5-Fc recombinant fusion protein and its new application
A fusion protein, sdr5-fc technology, applied in the direction of drug combination, peptide/protein composition, hybrid peptide, etc., to achieve the effect of improving survival rate, reducing liver cell apoptosis rate, and reducing liver pathological damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
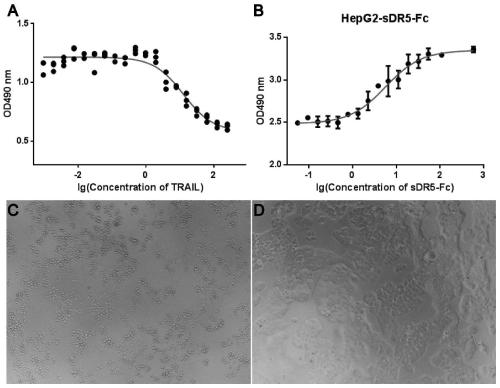
[0057] Example 1: Human sDR5-Fc recombinant fusion protein can block TRAIL-induced liver cell apoptosis
[0058] The HepG2 cells in the logarithmic growth phase were plated on a 96-well plate, and the same concentration of actinomycin D was added to each well to inhibit cell division, and 2-fold diluted TRAIL protein was added to each well to induce cell apoptosis. %CO 2 After incubating in the incubator for 18-22 hours, add freshly prepared 20:1 mixed MTS / PMS chromogenic solution 20ul / well, continue culturing in the incubator for 3-4 hours, and detect its A490-A630 with a microplate reader value. Thus, the EC90 value of TRAIL killing activity was calculated.
[0059] HepG2 cells in the logarithmic growth phase were plated on a 96-well plate, the same concentration of actinomycin D was added to each well to inhibit cell division, and the same concentration of TRAIL protein was added to each well to induce 90% cell apoptosis. 2-fold diluted human sDR5-Fc recombinant fusion p...
Embodiment 2
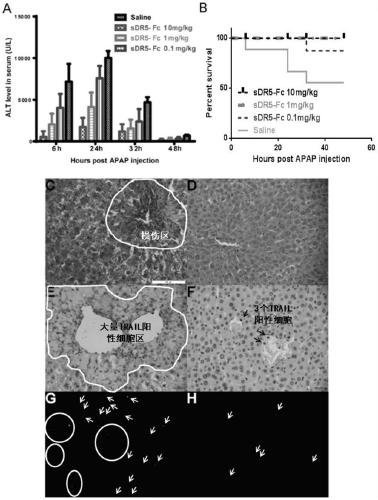
[0061] Example 2: Human sDR5-Fc antibody fusion protein in the treatment of APAP-induced liver injury
[0062] (1) 40 C57BL / 6 male mice were equally divided into 4 groups (normal saline group, 0.1mg / kg sDR5-Fc group, 1mg / kg sDR5-Fc group, 10mg / kg sDR5-Fc group, sDR5-Fc The sequence is SEQ ID NO.1), 10 mice in each group, each mouse was given 400mg / kg APAP by intragastric administration, and after 1 hour, each group of mice was given intraperitoneal injection of normal saline, 0.1mg / kg sDR5-Fc, 1mg / kg sDR5-Fc, 10mg / kg sDR5-Fc, the administration volume is 10ml / kg, 6h, 24h, 32h and 48h after APAP administration, blood was collected from the submandibular vein of each mouse, the serum was separated, and the serum was tested Transaminase levels. The mice were sacrificed at 48 hours, part of the liver was fixed in 4% PFA, embedded in paraffin, sectioned, HE stained, TUNEL stained, and TRAIL immunohistochemical stained.
[0063] The results are displayed (see figure 2 ): 10mg / kg...
Embodiment 3
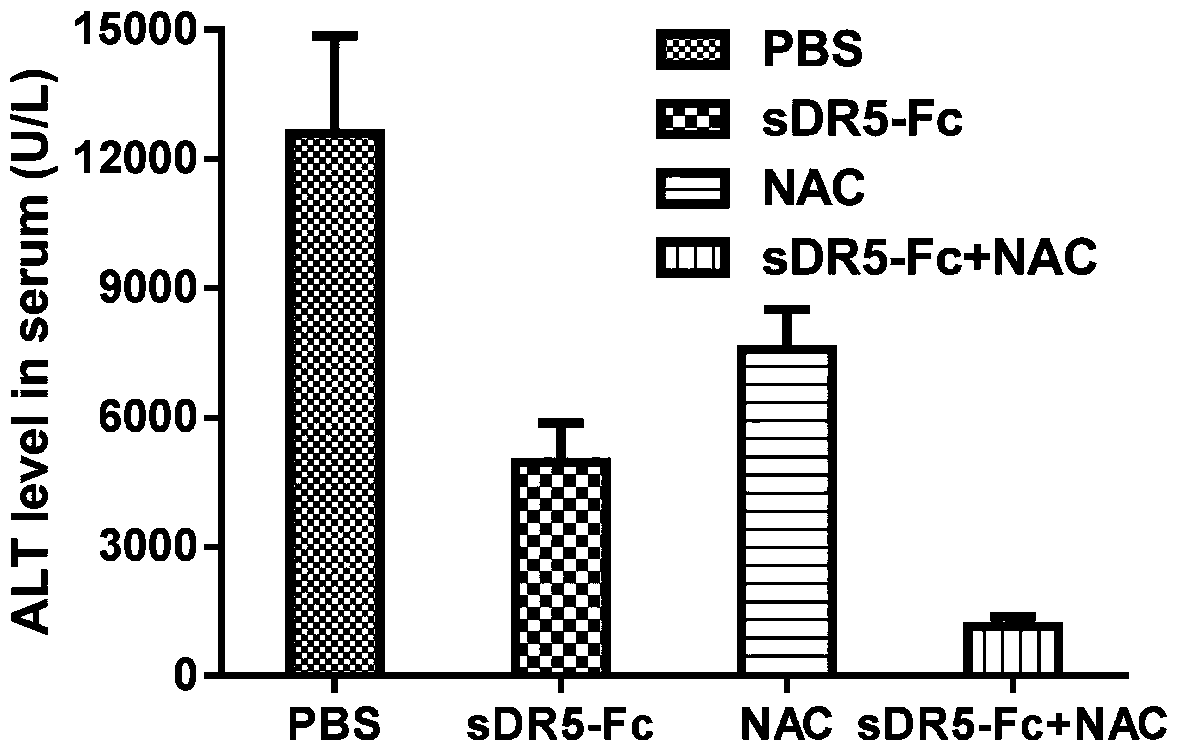
[0066] Example 3: Synergistic treatment of APAP-induced liver injury by human sDR5-Fc recombinant fusion protein and NAC
[0067] Forty C57BL / 6 male mice were equally divided into 4 groups (normal saline group, 10mg / kg sDR5-Fc group, 100mg / kgNAC group, 10mg / kg sDR5-Fc and 100mg / kg NAC combined administration group, sDR5-Fc The sequence is SEQ ID NO.1), 10 mice in each group, each mouse was given 500mg / kg APAP by intragastric administration, and after 1 hour, each group of mice was given intraperitoneal injection of normal saline, 10mg / kg sDR5-Fc , 100mg / kg NAC, 10mg / kg sDR5-Fc and 100mg / kg NAC were administered in combination, and the administration volume was 10ml / kg. 24 hours after APAP administration, blood was collected from the submandibular vein of each mouse, and the serum was separated and detected. Serum transaminase levels.
[0068] The results are displayed (see image 3 ): 10mg / kg sDR5-Fc recombinant fusion protein and 100mg / kg NAC can act synergistically to sign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com