Surface modification method of catalyst used for fuel cells
A technology of fuel cells and catalysts, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of reduced electrochemical active area, increased overpotential, platinum nanoparticle poisoning, etc., to facilitate large-scale production and simple method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Take 600 μL of 120.0 mM HO 2 PtCl 6 The aqueous solution was placed in a three-necked flask, and 55.0 mg of cetyltrimethylammonium bromide, 60.0 mg of ascorbic acid, and 60.0 ml of deionized water were added, and stirred to make the solution evenly mixed.
[0028] 2. Transfer the above solution to an oil bath at 100° C., heat and stir for 3 hours, add 5.3 mg of silver nitrate, react in an oil bath at 100° C. for 2 hours, and cool to room temperature.
[0029] 3. Add 50.0 mg of XC72 to the above solution, ultrasonically disperse it evenly, stir at room temperature for 12 hours, centrifuge, wash and dry.
[0030] 4. Mix 5 mg of the above catalyst with 2.5 ml of isopropanol and 20 μL of 5% Nafion aqueous solution, ultrasonically disperse the catalyst and Nafion in isopropanol evenly, take 6 μL of the suspension and apply it to a glassy carbon electrode, and use it as a working solution in a three-electrode system electrode, a platinum sheet as the counter electrode, a...
Embodiment 2 5
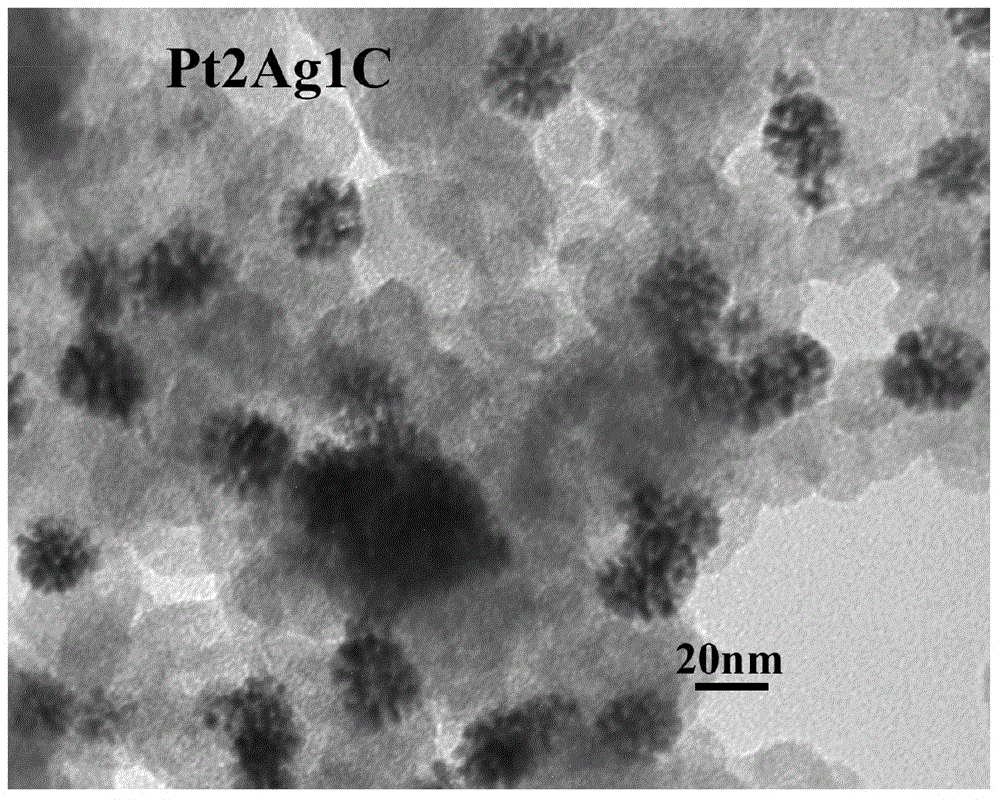
[0039] The steps are the same as in Example 1, except that the quality of silver nitrate added is 1.1 mg, 2.2 mg, 3.5 mg, and 11.0 mg, respectively, which are recorded as Pt 10 Ag 1 C. Pt 5 Ag 1 C. Pt 3 Ag 1 C. Pt 1 Ag 1 c.
[0040] Figure 6 for Pt 1 Ag 1 TEM image of C.
[0041] Figure 7 for Pt 10 Ag 1 C. Pt 5 Ag 1 C. Pt 3 Ag 1 C. Pt 2 Ag 1 C and Pt 1 Ag 1 XRD pattern of C before electrochemical treatment.
Embodiment 6
[0043] 1. Take 600 μL of 120.0 mM K 2 PtCl 4 The aqueous solution was placed in a three-necked flask, and 55.0 mg of cetyltrimethylammonium chloride, 60.0 mg of ascorbic acid, and 60.0 ml of deionized water were added, and stirred to make the solution evenly mixed.
[0044] 2. Transfer the above solution to an oil bath at 80°C, heat and stir for 3 hours, add 5.2mg CuSO 4 , reacted in an oil bath at 80°C for 2 hours, and cooled to room temperature.
[0045] 3. Add 50 mg of XC72 to the above solution, ultrasonically disperse it evenly, stir at room temperature for 12 hours, centrifuge, wash and dry.
[0046] 4. Mix 5.0 mg of the above catalyst with 2.5 ml of isopropanol and 20 μL of 5% Nafion aqueous solution, ultrasonically disperse the catalyst and Nafion in isopropanol evenly, take 6 μL of the suspension and apply it to a glassy carbon electrode, and use it as a three-electrode system Working electrode, platinum sheet as counter electrode, saturated calomel electrode as re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com