Drug composition for controlling and treating fatty liver of human body and application thereof
A technology of drugs and pharmaceutical preparations, applied in the field of traditional medicine, can solve the problems of unstable quality and huge difference of licorice extract, achieve stable quality and improve the effect of fatty liver symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
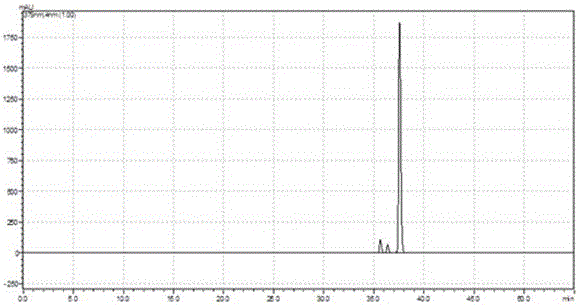
[0036] Example 1 Preparation of Licorice Extract
[0037] Take 50g of licorice, add 250mL of water, soak at 85°C for 7 hours, cool to room temperature, filter with filter paper, discard the filtrate; add 250ml of water to the filter residue, soak at 85°C for 7 hours, cool to room temperature, filter with filter paper, and discard the filtrate; Then, add 250 mL of water to the filter residue, soak at 85°C for 7 hours, cool to room temperature, filter with filter paper, and discard the filtrate.
[0038] Then, add 200 mL of 95% (v / v) ethanol to the filter residue, and extract at 85°C under reflux for 2 hours, cool to room temperature, filter with filter paper, and retain the filtrate; add 200 mL of 95% (v / v) ethanol to the filter residue and extract at 85°C under reflux For 2 hours, cool to room temperature, filter with filter paper, and retain the filtrate; then, add 200 mL of 95% (v / v) ethanol to the filter residue, reflux for extraction at 85°C for 2 hours, cool to room temperatur...
Embodiment 2
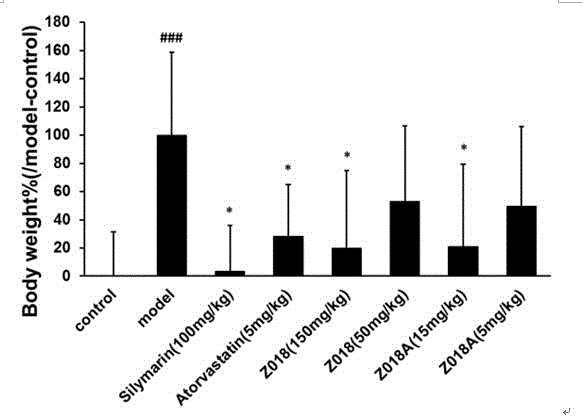
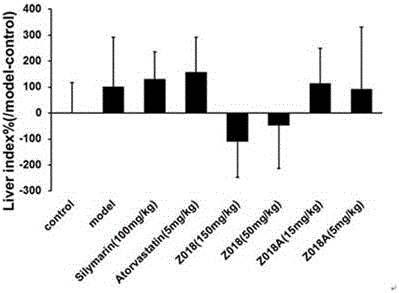
[0045] Example 2 Pharmacological test of licorice crude extract A and licorice extract on non-alcoholic fatty liver injury in rats
[0046] The crude licorice extract A (Z018) and licorice extract (Z018A) prepared in Example 1 were used as drugs for the high and low dose groups of Z018 and Z018A, respectively; the positive control group was administered with silymarin and atorvastatin, respectively drug. Each drug was dissolved with 0.5% CMC-Na.
[0047] Wistar rats (male, weighing 180g) were adaptively reared for one week and were randomly divided into normal control group (control), model group (model), silymarin control group (silymarin), atorvastatin control group (atorvastatin), Z018 high dose group, Z018 low dose group, Z018A high dose group, Z018A low dose group. The normal control group was fed basic feed (basic feed purchased from Beijing Keyao Xieli Feed Co., Ltd., batch number: 13083111), and the other groups were fed high-fat feed (78.8% basic feed + 1% cholesterol + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com