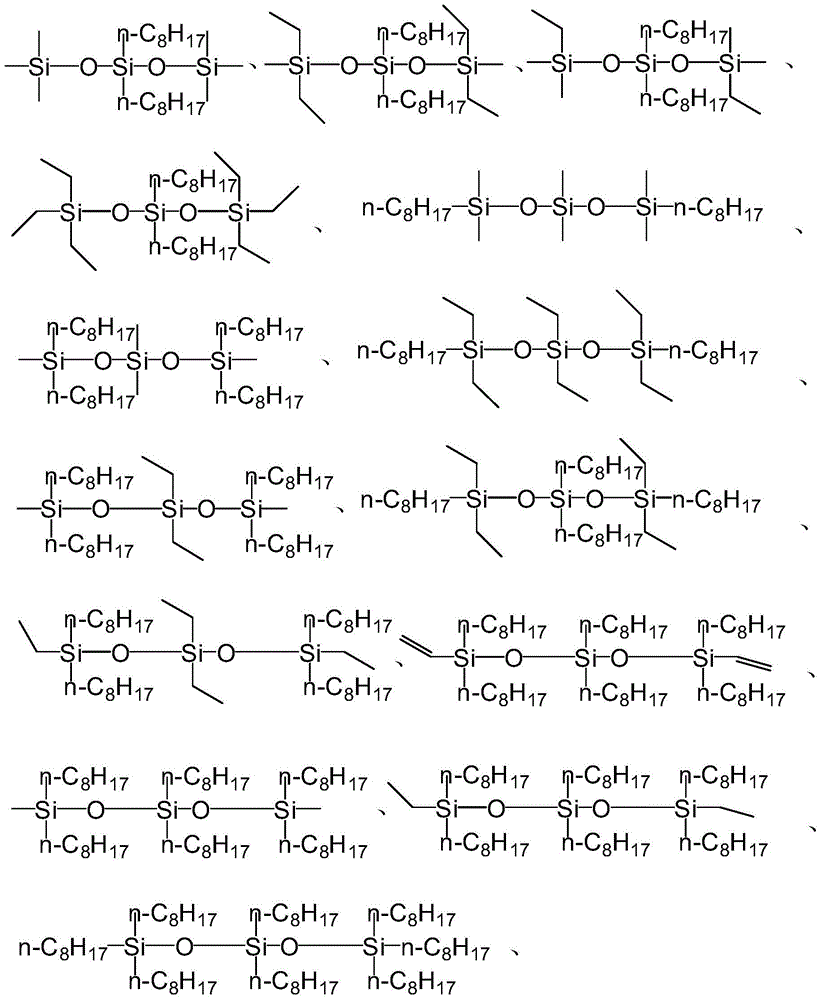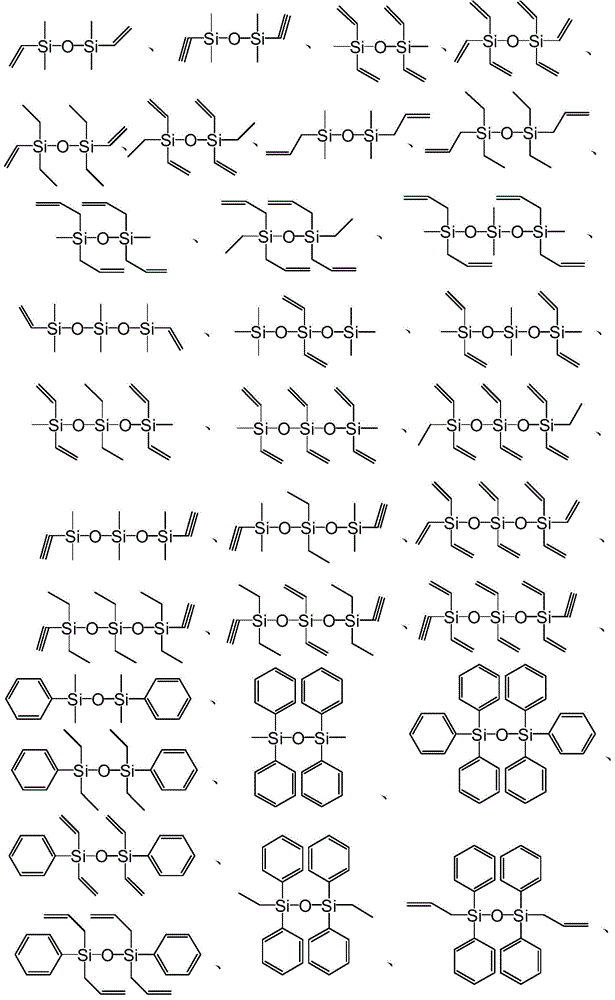Preparation method of difluorophosphate
A technology of difluorophosphate and fluoride salt, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low yield and low purity of crude products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] A kind of preparation method of difluorophosphate, is characterized in that, comprises the following steps:
[0054] A) mixing and stirring the non-aqueous solvent, the fluoride salt and the compound having the structure of formula I to obtain a suspension;
[0055] B) Passing PF into the suspension 5 After the gas is heated and reacted, it is recrystallized to obtain difluorophosphate;
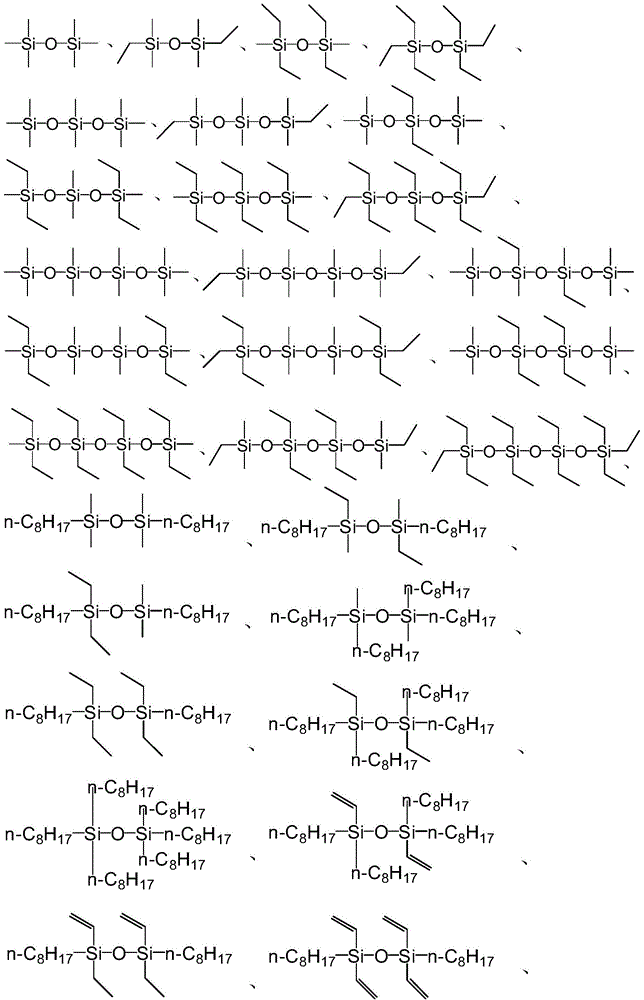
[0056] Formula I;
[0057] In formula I, R 1 ~R 6 are independently selected from H, C1-C50 hydrocarbon groups, C1-C50 substituted hydrocarbon groups or groups with the structure of formula II; or, R 1 ~R 6 Any two or more groups in are combined with each other to form a ring structure;
[0058] Formula II;
[0059] In formula II, R 7 ~R 9 are independently selected from H, C1-C50 hydrocarbon groups, C1-C50 substituted hydrocarbon groups, or R 7 ~R 9 Any one or more groups in are further substituted by a group having the structure of formula II.
[0060] In the present ...
Embodiment 1~22
[0122] Embodiment 1-22 is described in detail, and its specific experimental operation steps are: adding fluoride salt, a compound having the structure of formula I and a non-aqueous solvent into a dry closed container protected by an inert gas, and mixing and stirring to form a suspension , then lower the temperature to a low temperature (10°C), and pass a certain amount of PF at this temperature 5 Then heat up to 60°C to react for 12 hours. After the reaction, cool down to room temperature, filter to remove unreacted fluoride salt and other insoluble impurities, then remove the solvent by rotary evaporation, and then obtain high-purity difluorophosphate by recrystallization , filtered to remove the recrystallization solvent (acetone: anhydrous ether = 1:4, v / v), the obtained crystals were dried under reduced pressure at 80°C for 6 hours, the obtained white powder was weighed, and the yield was calculated. The results are shown in Table 1 for the reaction conditions and resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com