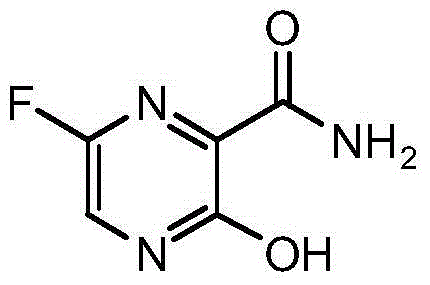
Preparation method for 6-fluoro-3-hydroxyl-2-pyrazinamide
The technology of pyrazinamide and hydroxyl group is applied in the field of preparation of 6-fluoro-3-hydroxy-2-pyrazinamide, can solve the problems of complicated steps, low yield and the like, and achieves simple and convenient preparation method, high yield and easy The effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Under nitrogen protection, dissolve 14g (64.5mmol, 1eq) of 6-bromo-3-amino-2-pyrazinamide in 200ml of N,N-dimethylformamide, and add 21g ( 96.75mmol, 1.5eq) Boc 2 O and 3.1g (129mmol, 2eq) of NaH were stirred at 25°C for 3h, the reaction was detected by TLC as complete, and the reaction was stopped. Then 5 times the volume of water was added under ice bath to quench the excess NaH, and at the same time, solids were precipitated in the system, filtered, and the filter cake was vacuum-dried at 25°C to obtain 16.6g of 6-bromo-3-carbamate tert-butyl ester-2- Pyrazinamide, the yield was 81%. The NMR index of 6-bromo-3-tert-butyl carbamate-2-pyrazinamide is as follows:
[0024] 1 H-NMR (DMSO-d6, 600MHz) δ value: 1.39 (9H, s, Boc), 7.72 (1H, s, NH 2 ),7.98(1H,s,NH 2 ), 8.47 (1H, s, pyrazine H), 9.82 (1H, s, NH).
[0025] (2) Under nitrogen protection, dissolve 2g (6mmol) of tert-butyl 6-bromo-3-carbamate-2-pyrazinamide in 20ml of dimethyl sulfoxide, and add 0.87g (15...
Embodiment 2
[0032](1) Under nitrogen protection, dissolve 0.50g (2.31mmol, 1eq) of 6-bromo-3-amino-2-pyrazinamide in 5ml of N,N-dimethylformamide, and add 0.76 Boc of g (3.47mmol, 1.5eq) 2 O and 0.11 g (4.62 mmol, 2 eq) of NaH were stirred at 30° C. for 3 h, and the reaction was detected to be complete by TLC, so the reaction was stopped. Then 5 times the volume of water was added under ice bath to quench the excess NaH, and at the same time, solids were precipitated in the system, filtered, and the filter cake was vacuum-dried at 30°C to obtain 0.57g of 6-bromo-3-carbamic acid tert-butyl ester- 2-Pyrazinamide, yield 78%. The NMR index of 6-bromo-3-tert-butyl carbamate-2-pyrazinamide is as follows:
[0033] 1 H-NMR (DMSO-d6, 600MHz) δ value: 1.37 (9H, s, Boc), 7.69 (1H, s, NH 2 ),7.94(1H,s,NH 2 ),8.43(1H,s,pyrazineH),9.80(1H,s,NH)
[0034] (2) Under nitrogen protection, dissolve 0.2g (0.63mmol) of 6-bromo-3-carbamic acid tert-butyl ester-2-pyrazinamide in 5ml of dimethyl sulfoxide, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com