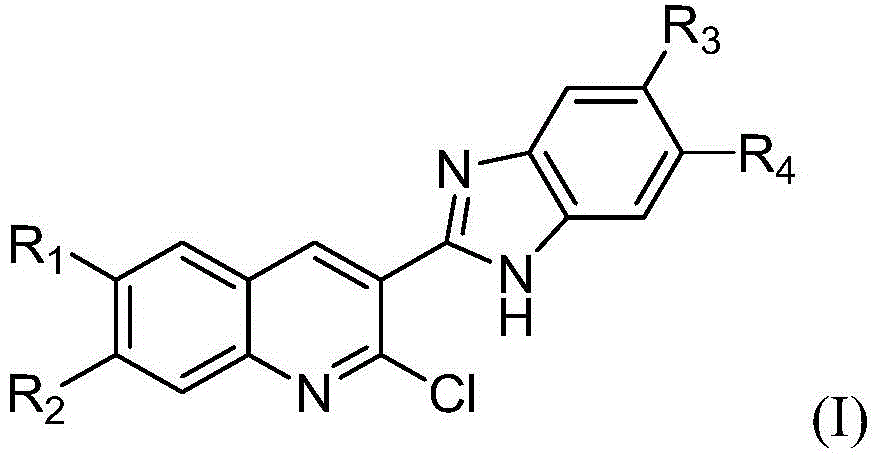
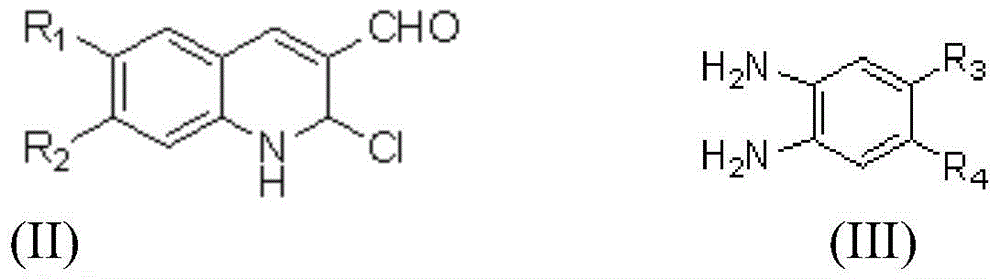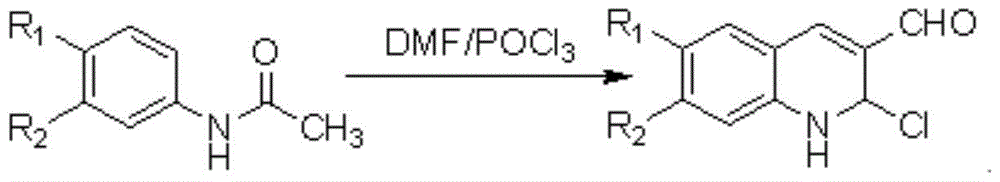2-chlorine-3-(1H-benzimidazole-2-radical)-quinoline derivative and preparation method and application thereof
A chlorine-based and methyl-based technology, applied in the field of medicine, achieves the effects of simple post-processing, improved anti-tumor activity, and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 1. Preparation of Compound 1: Mix 3.5mL of DMF and 17mL of POCl 3 After mixing and stirring evenly, 2.03 g of acetanilide was added, the temperature was raised to 90° C., heated to reflux for 16 hours, cooled and poured into a large amount of ice water, and filtered to obtain compound 1 with a yield of 88%.
[0034] Compound 1: 1 HNMR (500MHz, DMSO-d 6)δ: 10.49(s,1H,CHO),8.79(s,1H,C=CH),8.11(d,J=7.9Hz,1H,Ar–H),7.95(t,J=7.8Hz,1H, Ar–H),7.89(d,J=8.3Hz,1H,Ar–H),7.68(t,J=8.0Hz,1H,Ar–H); MSm / z:192[M+H] + .
[0035] 2. Preparation of compound 2: Referring to the synthetic procedure of compound 1, p-methylacetanilide was used instead of acetanilide to obtain compound 2 with a yield of 85%.
[0036] Compound 2: 1 HNMR (500MHz, DMSO-d 6 )δ:10.61(s,1H,CHO),8.80(s,1H,C=CH),8.12(d,J=7.8Hz,1H,Ar–H),8.01(t,J=7.9Hz,1H, Ar–H),7.75(d,J=8.3Hz,1H,Ar–H),2.61(s,CH3); MSm / z:206[M+H] + .
[0037] 3. Preparation of compound 3: Referring to the synthesis procedure of compound 1, p-meth...
Embodiment 1
[0041] Example 1: Preparation of 2-chloro-3-(5,6-dimethyl-1H-benzimidazol-2-yl)-6-methoxyquinoline (compound a)
[0042] Weigh 30.2g (0.90mmol) of the compound, 0.122g (0.90mmol) of 4,5-dimethyl-o-phenylenediamine, and 6mL of anhydrous methanol into a pressure-resistant test tube (sealed), and stir the reaction at 80°C To complete (TLC tracking detection, about 9h), after cooling, filter with suction and wash with absolute ethanol to obtain 0.179 g of compound a (light yellow solid), with a yield of 59%.
[0043] Compound a: Yields59%, 1 HNMR (500MHz, DMSO-d 6 )δ12.88(s,1H),8.84(s,1H),7.96(d,J=9.2Hz,1H),7.60(s,1H),7.55(d,J=9.1Hz,1H),7.46( s,2H),3.93(s,3H),2.36(s,6H); 13 CNMR (126MHz, DMSO-d 6 )δ158.70, 147.27, 144.89, 143.27, 140.29, 131.65, 129.64, 128.16, 125.06, 124.67, 106.81, 56.24, 20.49. MSm / z: 338[M+H] + .
[0044] Therefore, it can be determined that the above-mentioned compound a is 2-chloro-3-(5,6-dimethyl-1H-benzimidazol-2-yl)-6-methoxyquinoline, and its stru...
Embodiment 2
[0046] Example 2: 2-chloro-3-(5,6-dimethyl-1H-benzimidazol-2-yl)-[1,3]dioxolane[6,7-g]quinoline ( Preparation of compound b)
[0047] Weigh 40.2g (0.85mmol) of the compound, 0.116g (0.85mmol) of 4,5-dimethyl-o-phenylenediamine, and 6mL of anhydrous methanol into a pressure-resistant test tube (sealed), and stir the reaction at 80°C To complete (TLC tracking detection, about 7.5h), after cooling, filter with suction, wash with absolute ethanol to obtain 0.192g of compound b (light yellow solid), with a yield of 64.5%.
[0048] Compound b: Yields64.5%, 1 HNMR (500MHz, DMSO-d 6 )δ12.19(s, 1H), 8.75(s, 1H), 7.54(s, 1H), 7.44(d, J=5.6Hz, 3H), 6.29(s, 2H), 2.35(s, 6H); 13 CNMR (126MHz, DMSO-d 6 )δ152.94, 149.07, 147.41, 145.96, 145.09, 140.00, 131.45, 124.15, 122.76, 104.53, 103.47, 103.20, 20.4. MSm / z: 352[M+H] + .
[0049] Therefore, it can be determined that the above compound b is 2-chloro-3-(5,6-dimethyl-1H-benzimidazol-2-yl)-[1,3]dioxolane[6,7-g ] quinoline, its structu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com