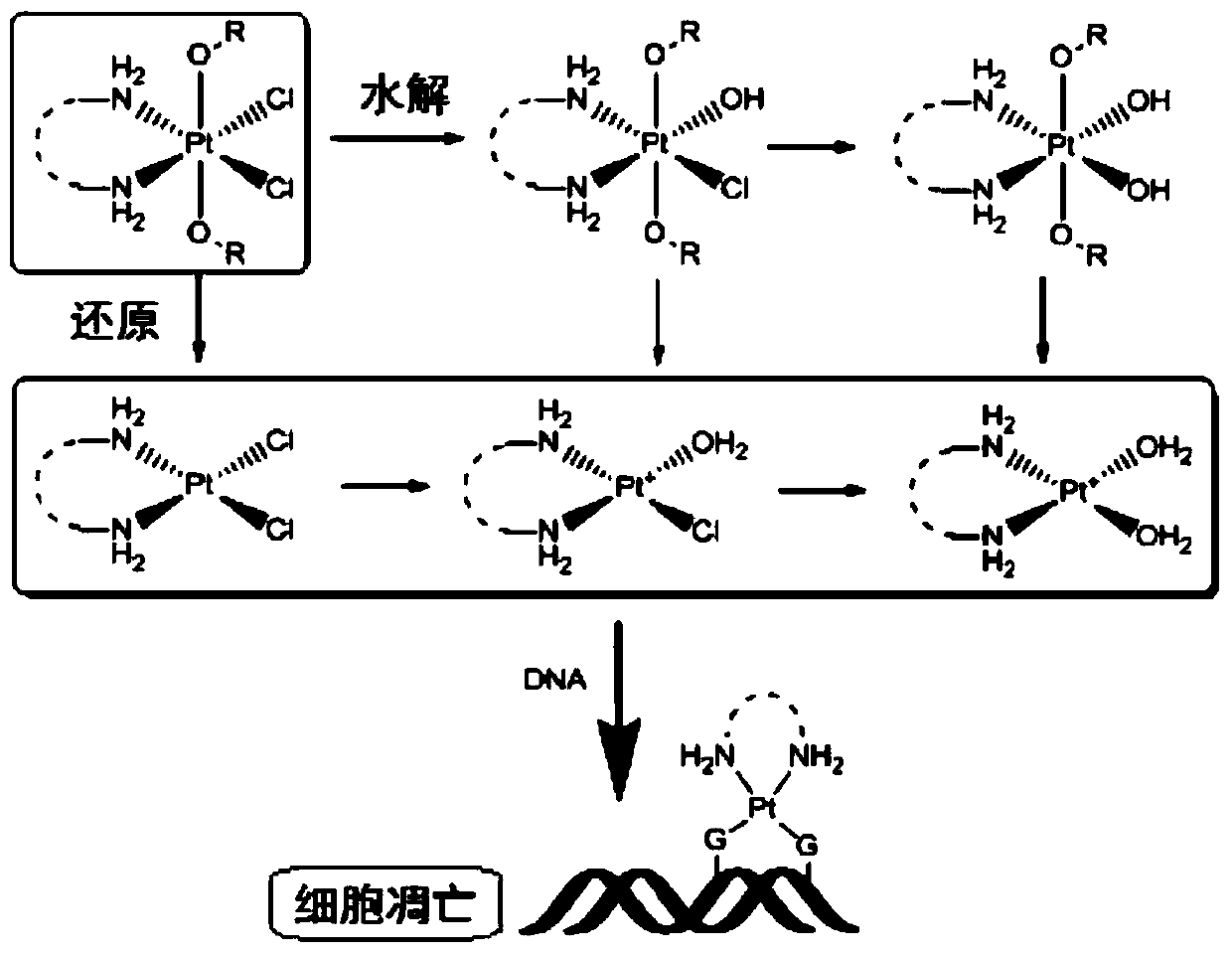
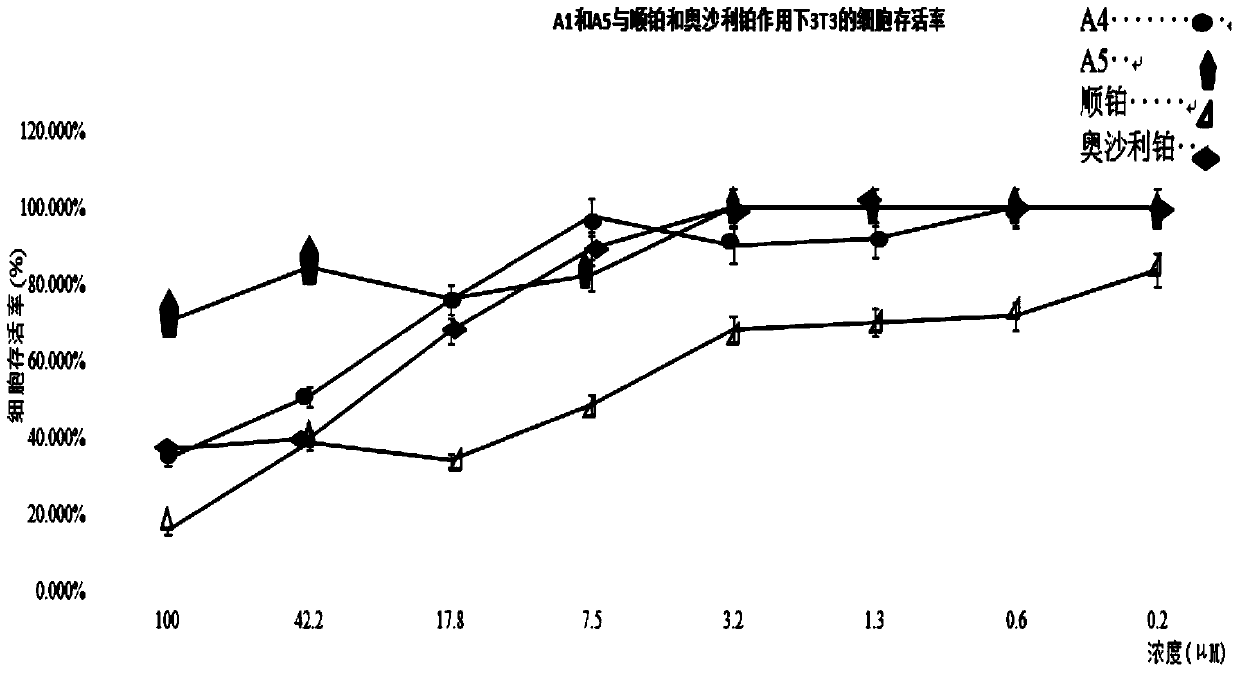
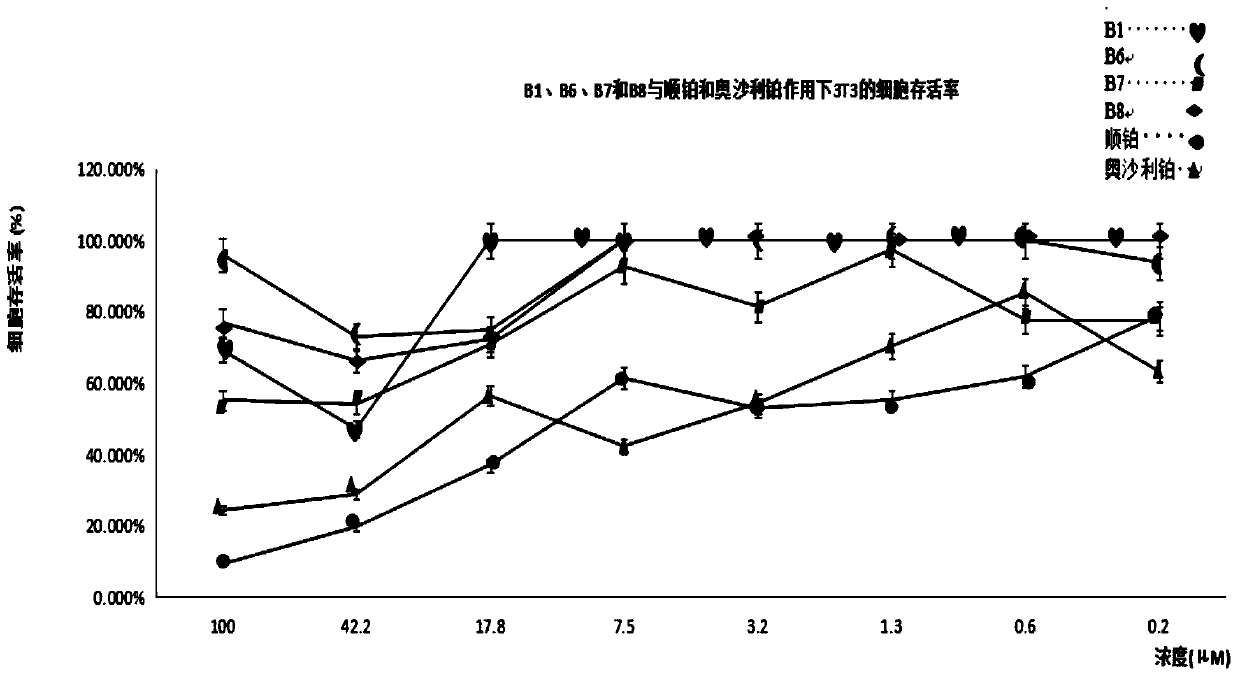
Tetravalent platinum glycosyl complex for tumor treatment and preparation method thereof
A tumor treatment, tetravalent platinum technology, applied in the preparation of sugar derivatives, medical preparations containing active ingredients, sugar derivatives, etc. The effect of improving ester-water partition coefficient, increasing maximum tolerated dose, and increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the synthesis of tetravalent cisplatin carboxylic acid
[0060]
[0061] C2 HRMS: Calcd.for Cl 2 h 8 N 2 o 2 Pt(M + ):332.96,found:332.9827.
[0062] C3 HRMS: Calcd.for C 4 h 12 Cl 2 N 2 o 5 Pt(M + ):432.98,found:432.9847.
Embodiment 2
[0063] Example 2: Synthesis of Glycosylated Tetravalent Platinum Final Product A1
[0064]
[0065] The preparation method is as follows: C1 (divalent platinum compound cisplatin) is oxidized with hydrogen peroxide at 60-70°C and reacted for 4 hours to prepare C2 tetravalent cisplatin compound; C2 (1equiv) and succinic anhydride ( 4equiv) was dissolved in DMF, reacted overnight at 70°C under nitrogen protection, and then the oil pump was drained. After adding dichloromethane to precipitate a solid, C4 could be prepared by washing with chloroform, ether, etc.; the DMF solution of C4 was mixed with 2-(7 -Azobenzotriazole)-N,N,N',N'-Tetramethyluronium hexafluorophosphate DMF solution was mixed and stirred for 10 minutes, then added full acetylated glucosamine and N,N-di The DMF mixture of isopropylethylamine was reacted at room temperature in the dark for 24 hours to obtain A1.
[0066] A1: 1 H NMR (400MHz, CDCl 3 )δ6.41(d,J=146.8Hz,4H),5.47–4.79(m,14H),4.64–4.08(m,5H),4.02...
Embodiment 3
[0067] Example 3: Synthesis of Glycosylated Tetravalent Platinum Final Product A2
[0068]
[0069] The preparation method is as follows: C1 (divalent platinum compound cisplatin) is oxidized with hydrogen peroxide at 60-70°C and reacted for 8 hours to prepare C2 tetravalent cisplatin compound; C2 (1equiv) and succinic anhydride ( 4equiv) was dissolved in DMF, reacted overnight at 70°C under nitrogen protection, and then the oil pump was drained. After adding dichloromethane to precipitate a solid, C4 could be prepared by washing with chloroform, ether, etc.; the DMF solution of C4 was mixed with 2-(7 -Azobenzotriazole)-N,N,N',N'-Tetramethyluronium hexafluorophosphate DMF solution was mixed and stirred for 20 minutes, then added full acetylated rhamnosamine and N,N - DMF mixed solution of diisopropylethylamine, A2 was obtained after reaction at room temperature in the dark for 48 hours.
[0070] A2: 1 H NMR (400MHz, CDCl 3 )δ5.28(s,6H),5.22–4.89(m,5H),4.72(s,1H),3.78(d,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com