Metronidazole gel free of preservative and preparation method thereof
A preservative and metronidazole technology, applied in the field of biomedicine, can solve the problems of difficult long-term control, easy recurrence of the disease, misdiagnosed as bacterial blepharitis, etc., and achieves the effects of good stability and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
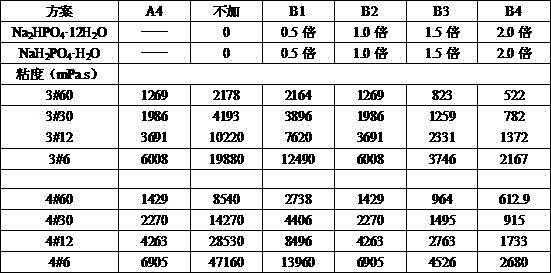
[0015] A preservative-free metronidazole ophthalmic gel is prepared by the following steps: (a) after dissolving 0.2% carbomer and 0.2% hypromellose in 10% water according to the proportion, Add 0.2% triethanolamine to the carbomer solution and stir until clear, then add the dissolved hypromellose solution, mix well, record it as liquid A, and set aside; (b) mix 0.08% disodium hydrogen phosphate, 0.02 % sodium dihydrogen phosphate, 2.5% glycerin, and 0.05% metronidazole were added to an appropriate amount of water in turn, stirred evenly to dissolve, recorded as liquid B, and set aside; (c) Mix the two solutions A and B evenly, and the remaining Continue to make up to 100% with water, sterilize at 121°C for 30 minutes under hot pressure, and cool naturally to obtain the gel product.
Embodiment 2
[0017] A preservative-free metronidazole ophthalmic gel is prepared by the following steps: (a) after dissolving 0.1% carbomer and 0.1% hypromellose in 10% water respectively according to the proportion, Add 0.1% triethanolamine to the carbomer solution and stir until clear, then add the dissolved hypromellose solution, mix well, record it as liquid A, and set aside; (b) mix 0.05% disodium hydrogen phosphate, 0.02 % sodium dihydrogen phosphate, 1% glycerin, and 0.01% metronidazole were added to an appropriate amount of water in turn, stirred evenly to dissolve, recorded as B liquid, and set aside; (c) Mix the two solutions A and B evenly, and the remaining Continue to make up to 100% with water, sterilize at 121°C for 30 minutes under hot pressure, and cool naturally to obtain the gel product.
Embodiment 3
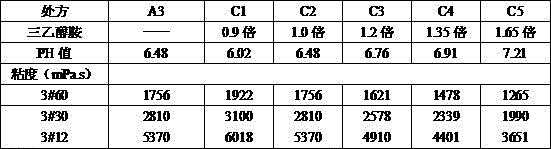
[0019] A preservative-free metronidazole ophthalmic gel is prepared by the following steps: (a) after dissolving 0.4% carbomer and 0.3% hypromellose in 15% water according to the proportion, Add 0.8% triethanolamine to the carbomer solution and stir until clear, then add the dissolved hypromellose solution, mix well, record it as liquid A, and set aside; (b) mix 0.2% disodium hydrogen phosphate, 0.1 % sodium dihydrogen phosphate, 2.5% glycerin, and 0.1% metronidazole were added to an appropriate amount of water in turn, stirred evenly to dissolve, recorded as liquid B, and set aside; (c) Mix the two solutions A and B evenly, and the remaining Continue to make up to 100% with water, sterilize under hot pressure at 121°C for 30 minutes, and cool naturally to obtain the gel product, which is designated as A3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com