Method for preparing cordyceps sinensis-containing pure powder tablets under low pressure
A technology for Cordyceps sinensis micropowder and Cordyceps sinensis Cordyceps sinensis is applied in the field of preparing qualified Cordyceps sinensis pure powder tablets, which can solve the problem of inability to prepare qualified Cordyceps sinensis pure powder tablets and the like, and achieve the effects of short disintegration time limit, large total pore area and high porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]The concrete preparation method of this example is as follows:
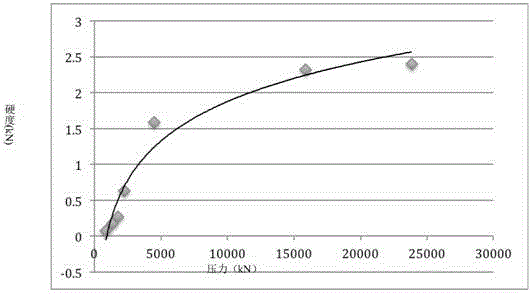
[0032] (1): Grinding the Cordyceps sinensis grass with a water content of less than 8% to a powder with a particle size of 48-75 μm, and pressing the Cordyceps sinensis powder under the condition of controlling the tableting pressure to 0.45kN by dry pressing at room temperature. Tablets with a diameter of 10 mm;
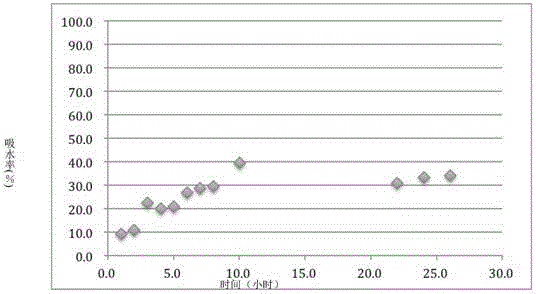
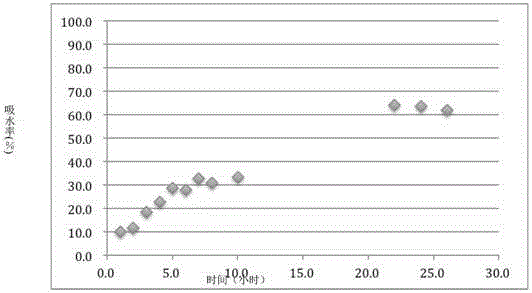
[0033] (2) the above-mentioned prepared Cordyceps tablet is placed in a water vapor environment with a vapor pressure of about 20kPa to absorb water for 12 hours, so that the adsorption capacity of the Cordyceps tablet to water is 27.2% of its own weight;
[0034] (3) Immediately put the Cordyceps sinensis tablet after water absorption into the environment of -5 ℃ and freeze for 10 hours, until all the liquid water in the sample forms solid ice;
[0035] (4) Freeze-dry the frozen tablet at a temperature of -25° C. until constant weight.
[0036] Sample analysis:
[0037] 1. Linear inspection o...
Embodiment 2
[0059] The concrete preparation method of this example is as follows:
[0060] (1): Grinding the Cordyceps sinensis grass with a water content of less than 8% to a powder with a particle size of 58-150 μm, and pressing the Cordyceps sinensis powder under the condition of controlling the tableting pressure to 2.25kN by dry pressing at room temperature. Tablets with a diameter of 10 mm.
[0061] (2) The Cordyceps tablet prepared above was placed in a water vapor environment with a vapor pressure of about 15kPa to absorb water for 12 hours, so that the adsorption capacity of the Cordyceps tablet to water was 41.7% of its own weight.
[0062] (3) Immediately freeze the Cordyceps sinensis tablet after absorbing water in an environment of -5° C. for 12 hours, until all the liquid water in the sample forms solid ice.
[0063] (4) Freeze-dry the frozen tablet to a constant weight at a temperature set at -25°C.
[0064] Sample analysis:
[0065] 1. Tablet hardness test
[0066] Fir...
Embodiment 3
[0078] The concrete preparation method of this example is as follows:
[0079](1): Grinding the Cordyceps sinensis grass with a water content of less than 8% to a powder with a particle size of 25-38 μm, and pressing the Cordyceps sinensis powder under the condition of controlling the tableting pressure to 4.95kN by dry pressing at room temperature. Tablets with a diameter of 10 mm.
[0080] (2) The Cordyceps tablet prepared above was placed in a water vapor environment with a vapor pressure of about 10 kPa to absorb water for 12 hours, so that the adsorption capacity of the Cordyceps tablet to water was 22.7% of its own weight.
[0081] (3) Immediately freeze the Cordyceps sinensis tablet after absorbing water in a -5°C environment for 24 hours, until all the liquid water in the sample forms solid ice.
[0082] (4) Freeze-dry the frozen tablet to a constant weight at a temperature set at -25°C.
[0083] Sample analysis:
[0084] 1. Tablet hardness test
[0085] First adop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com