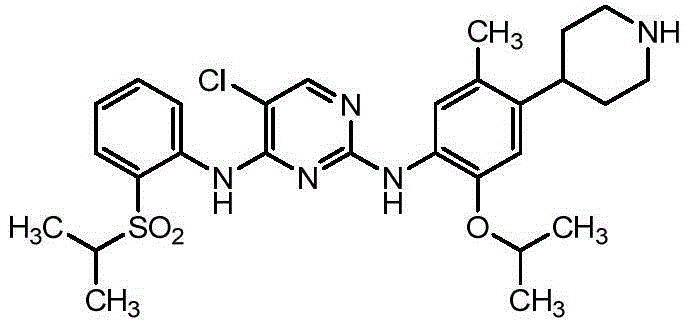
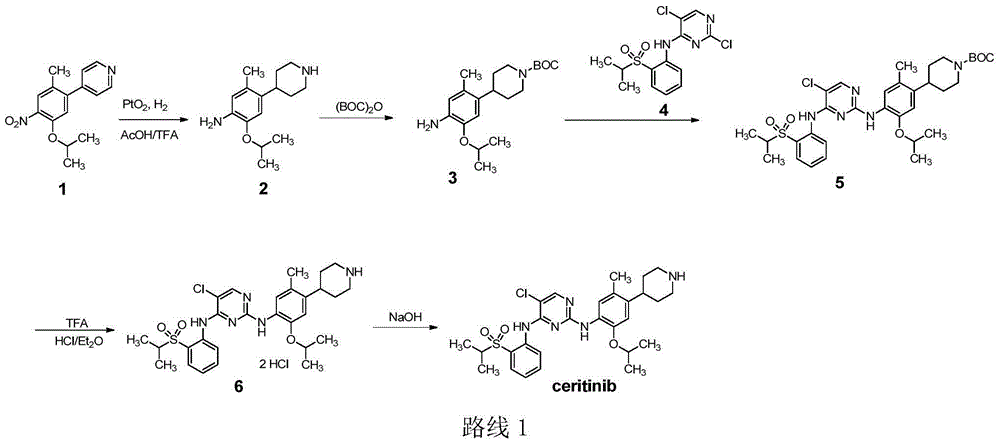
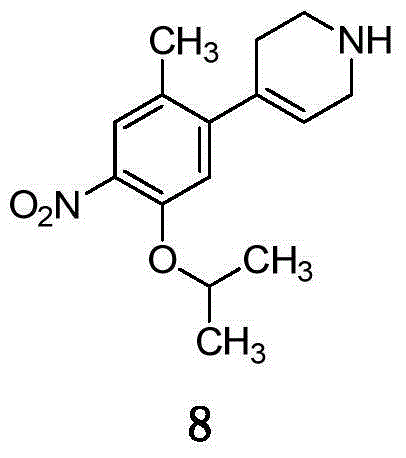
Ceritinib synthesis intermediate and preparation method thereof
A technology of ceritinib and intermediates, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, unsuitable for industrial production, difficult to complete the reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1. Synthesis of compound 7a
[0027]
[0028] Compound 1 (2.0g, 7.34mmol) and 20mL of ethyl acetate were put into the reaction flask, dissolved under stirring, and 6mL of hydrogen chloride in ethyl acetate solution (6M) was added dropwise. After the addition was completed and stirred for a period of time, a solid precipitated out. After filtration, a total of 2.0 g of compound 7a was obtained.
Embodiment 2
[0029] Embodiment 2. Synthesis of compound 7b
[0030]
[0031] Put compound 1 (2.0g, 7.34mmol) and 20mL ethyl acetate into the reaction flask, dissolve it under stirring, dissolve methanesulfonic acid (1.1g, 11.4mmol) in 3m; ethyl acetate, drop into the reaction flask After the dropwise addition was completed and stirred for a period of time, a solid precipitated out and was filtered to obtain a total of 2.6 g of compound 7a.
Embodiment 3
[0032] Embodiment 3. Synthesis of compound 8
[0033]
[0034] Put compound 7a (3.1g, 10.0mmol) and THF (30ml) into the reaction flask, under nitrogen protection, lower the temperature to below 5°C, add NaBH4 (0.38g, 10.0mmol) in batches, keep stirring below 5°C after the addition is complete . After the reaction, add 10ml of water dropwise. After the dropwise addition, evaporate THF under reduced pressure, add 100ml of ethyl acetate and 100ml of water to the residue, stir and separate layers, wash the ethyl acetate phase with water twice, and then wash with saturated saline. The organic phase was evaporated to dryness three times to obtain 2.5 g of compound 8 with a yield of 93.6%. MS: m / z=277; 1 HNMRδ(DMSO): 1.15-1.16 (6H, d), 3.30-3.48 (5H, m), 4.82-4.91 (2H, m), 6.08-6.10 (2H, m), 6.66-6.70 (1H, t), 7.62 (1H, s), 7.89 (1H, s), 8.33 (1H, d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com