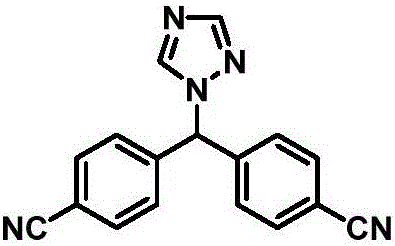
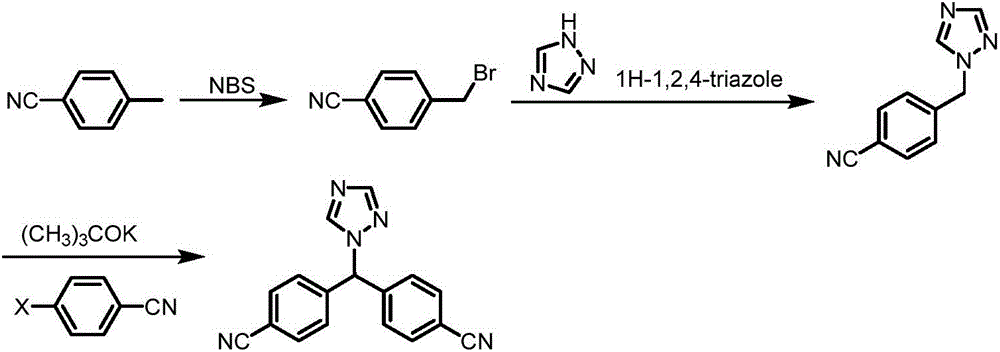
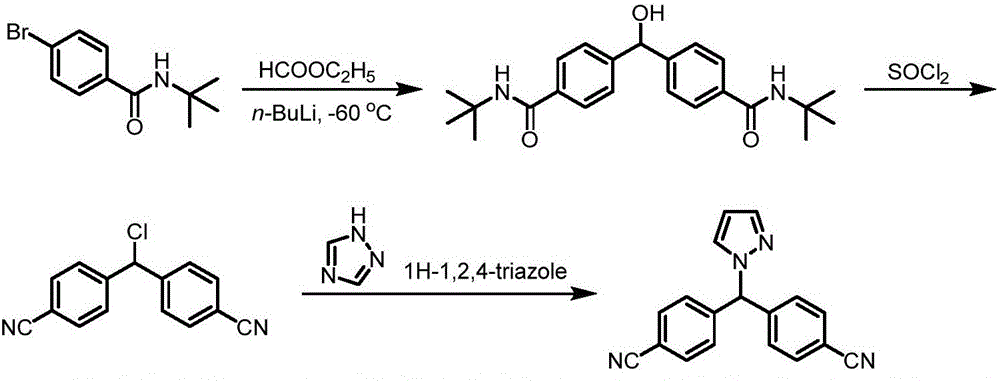
Preparation method of letrozole
A technology of letrozole and triazole, applied in the field of synthesis of highly selective third-generation aromatase inhibitors, can solve problems such as difficult large-scale industrial production, poor chemical selectivity, and harsh reaction conditions, and achieve The effect of low production cost, great application value and social and economic benefits, and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of 4,4-dicyanodiphenylmethane (compound 3)
[0050]Add 36g (0.32mol) of potassium tert-butoxide into 120ml of tetrahydrofuran, add dropwise a solution of 23.4g (0.2mol) of tetrahydrofuran (40ml) of p-methylbenzonitrile at -10°C, stir for half an hour after the addition, and keep the temperature constant Change. Weigh 32.9g (0.24mol) of p-chlorobenzonitrile, dissolve it in 80ml tetrahydrofuran, slowly drop it into the above reaction solution, control the temperature and react at -5~0°C, and continue the reaction for 2h after dropping. After the reaction, add water and stir to precipitate a solid, suction filter, wash the filter cake with water, and dry to obtain 31.6 g of a light yellow product with a yield of 72%.
[0051] Melting point: 167-169℃, 1 HNMR (400MHz, CDCl 3 )δ7.62(d, 4H, J=8.2Hz), 7.29(d, 4H, J=8.2Hz), 4.11(s, 2H).
Embodiment 2
[0052] Embodiment 2: Preparation of 4,4-dicyanodiphenylmethane (compound 3)
[0053] Dissolve 23.4g (0.2mol) of p-toluonitrile in 40ml of tetrahydrofuran, and add n-butyllithium in tetrahydrofuran (128ml, 2.5M / L) dropwise under cooling at -60°C, and continue stirring after the addition is complete For half an hour, at this temperature, a tetrahydrofuran (80ml) solution of 32.9g (0.24mol) of p-chlorobenzonitrile was added dropwise, and the reaction temperature was controlled at -60 to -50°C, and the reaction was continued for 2h after dropping. Then it was slowly raised to room temperature, and after the reaction was completed, water was added to precipitate a solid, filtered with suction, washed with water, and dried to obtain 35.1 g of a light yellow product with a yield of 80%.
[0054] Melting point: 167-169℃, 1 HNMR (400MHz, CDCl 3 )δ7.62(d, 4H, J=8.2Hz), 7.29(d, 4H, J=8.2Hz), 4.11(s, 2H).
Embodiment 3
[0055] Embodiment 3: Preparation of 4,4-dicyanodiphenyl bromide (compound 4)
[0056] In the reaction flask, add 11.0g (0.050mol) 4,4'-dicyanodiphenylmethane (compound 3), 9.8g (0.056mol) NBS, 303mg benzoyl peroxide and 50ml dichloromethane, stir Reflux, react for 6 hours, cool to room temperature, add water and stir, separate the organic phase, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill the filtrate to remove the solvent under reduced pressure to obtain 13.6 g of a yellow solid with a yield of 91.6%.
[0057] Melting point: 118-120°C. 1 HNMR (500MHz, CDCl 3 )δ7.68(d,4H,J=8.3Hz,7.55(d,4H,J=8.3Hz),6.25(s,1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com