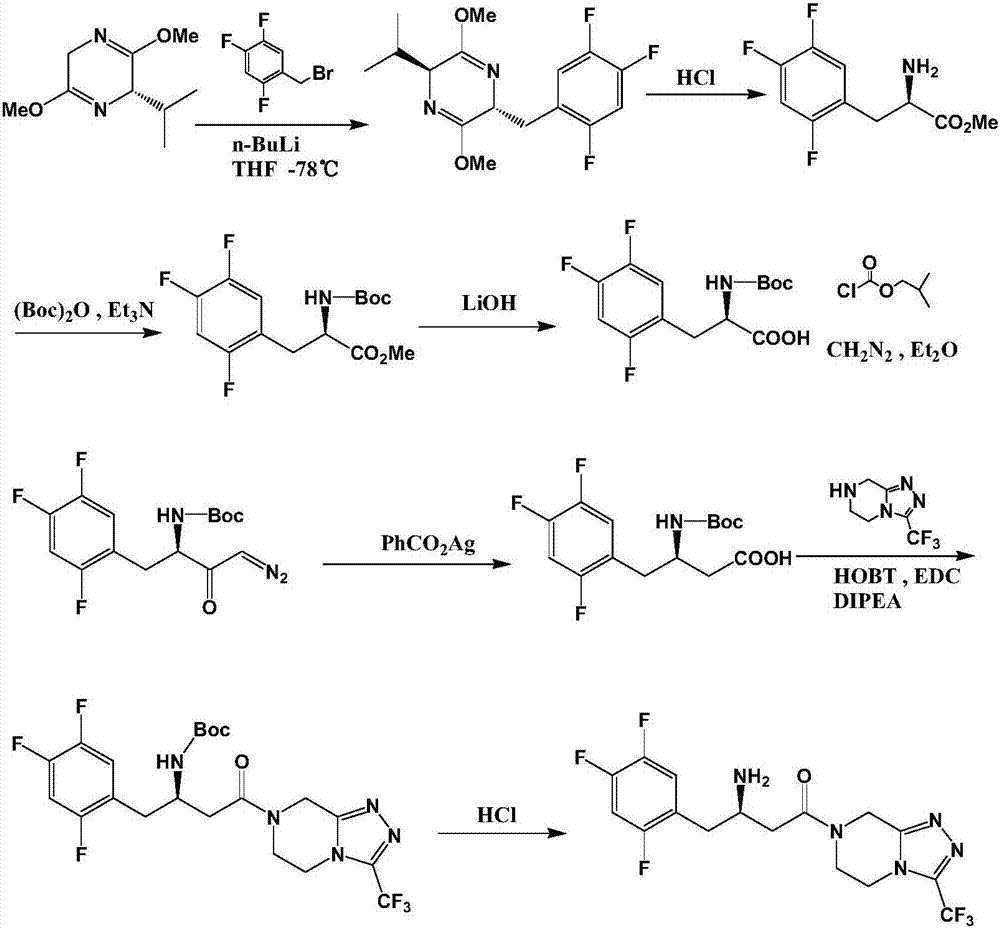
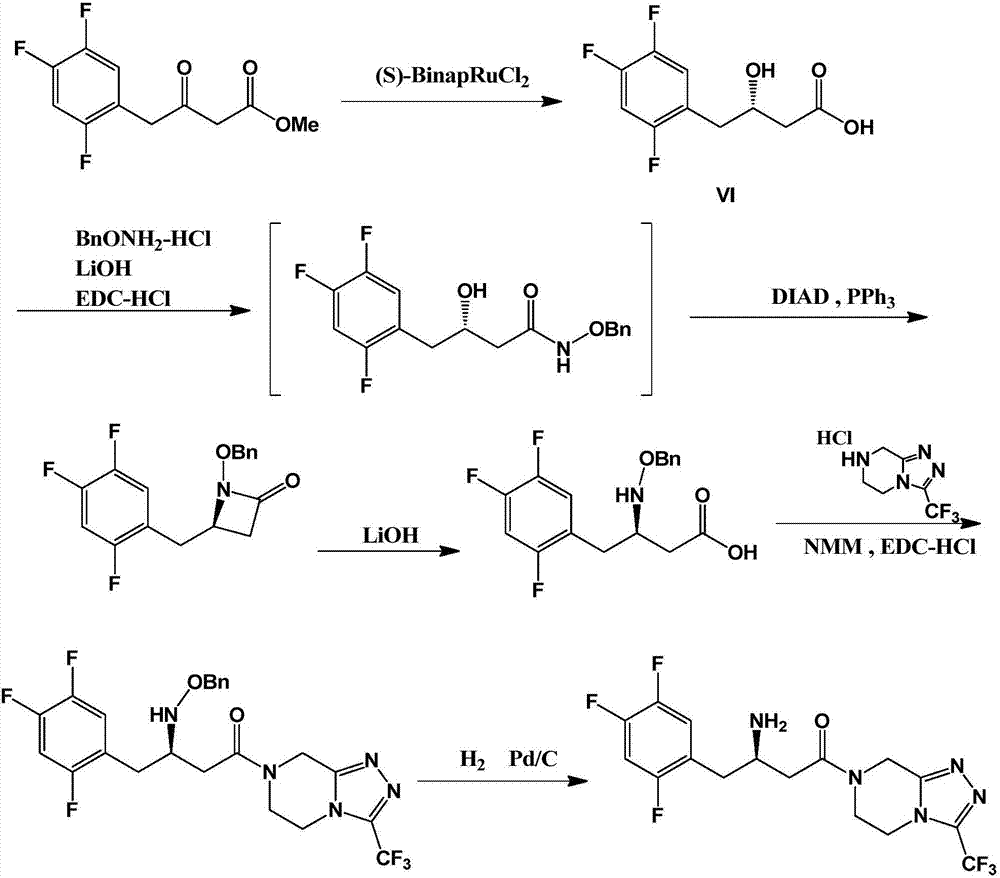
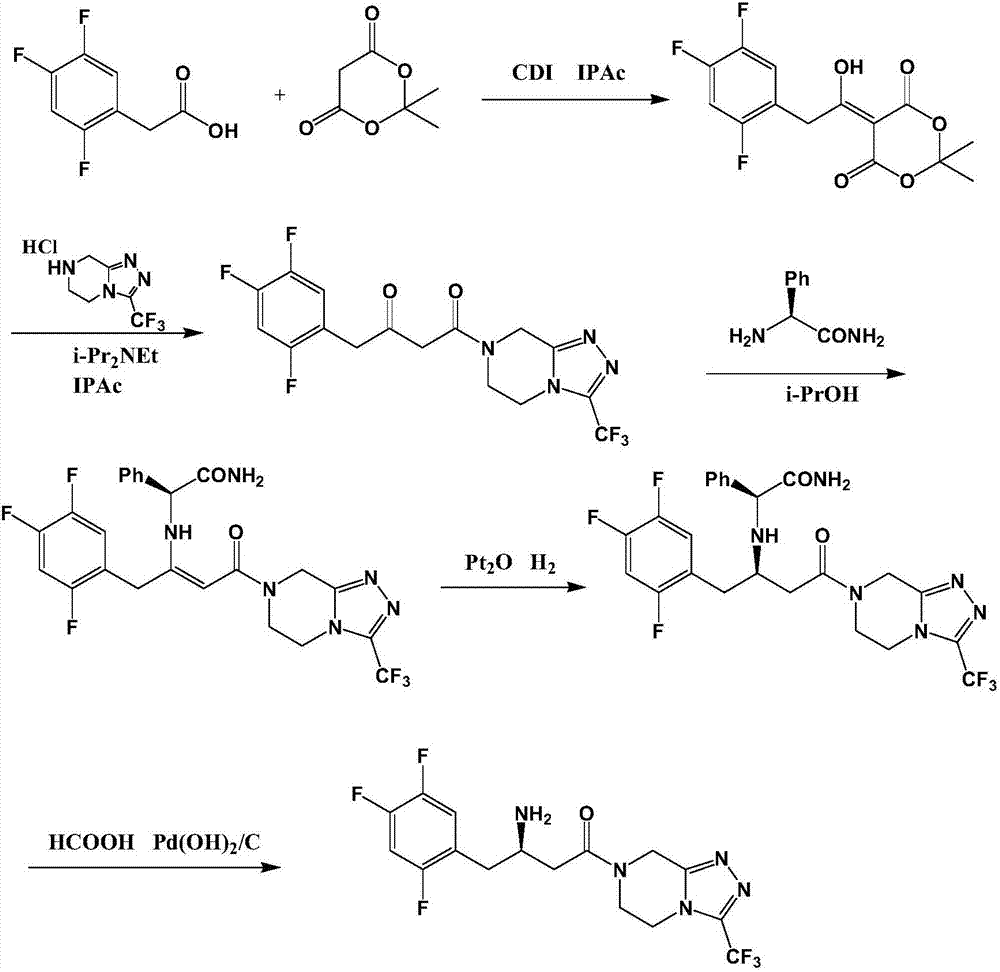
Preparation method of sitagliptin intermediate
A technology for sitagliptin and an intermediate is applied in the field of preparation of a sitagliptin intermediate for diabetes medicine, can solve problems such as restricting large-scale production, and achieve the effects of improving production efficiency, easy implementation and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Step 1. Preparation of (S)-(2,4,5-trifluorophenyl)-3-hydroxy-pentanone (compound III):
[0056] Add 298g of acetone, 800g of ethanol, and 115g of L-proline into the reaction flask, stir to dissolve, then slowly cool down to -40~-50°C, add 174.1g of 2,4,5-trifluorophenylacetaldehyde dropwise, about two The reaction was continued for about 8 hours, and the gas chromatography GC monitored that the content of the 2,4,5-trifluorophenylacetaldehyde system was less than 1%. The system was heated to -5°C, and 600ml of water and 700ml of ethyl acetate were added to separate The organic layer was dried by adding magnesium sulfate, and filtered. The obtained filtrate had a GC purity of 94% after subtracting the solvent ethyl acetate, and was directly used in the next reaction.
[0057] Step 2. Preparation of (S)-(2,4,5-trifluorophenyl)-3-tert-butyldimethylsilyloxy-butanone (compound IV):
[0058] Cool the ethyl acetate solution obtained in step 1 to 0°C, add 79.0 g of pyridine, a...
Embodiment 2
[0064] Step 1. Preparation of (S)-(2,4,5-trifluorophenyl)-3-hydroxy-pentanone (compound III):
[0065] Add 298g of acetone, 1000g of ethanol, and 170g of L-proline into the reaction flask, stir to dissolve, then slowly cool down to -40--50°C, add 174.1g of 2,4,5-trifluorophenylacetaldehyde dropwise, about two After hours of dripping, continue to react for about 10 hours. GC monitors that the content of 2,4,5-trifluorophenylacetaldehyde system is less than 1%. The system is heated to -5°C, 600ml of water and 700ml of ethyl acetate are added, and the organic layer is separated , added magnesium sulfate to dry, filtered, and the resulting filtrate had a GC purity of 95% of compound III after deducting the solvent ethyl acetate, and was directly used for the next step reaction.
[0066] Step 2~4.
[0067] Except that the starting reactant was replaced by the product of step 1 of this example, the remaining steps were carried out according to steps 2-4 of example 1.
[0068] Fina...
Embodiment 3
[0070] Add 240g of acetone, 700g of methanol, and 140g of L-proline into the reaction flask, stir to dissolve, then slowly cool down to -45--50°C, add 174.1g of 2,4,5-trifluorophenylacetaldehyde dropwise, about two After hours of dripping, continue to react for about 10 hours. GC monitors that the content of 2,4,5-trifluorophenylacetaldehyde system is less than 1%, and the system is heated to -5°C. Add 500ml of water and 700ml of ethyl acetate, and separate the organic layer , added magnesium sulfate to dry, filtered, and the resulting filtrate had a GC purity of 94% of compound III after deducting the solvent ethyl acetate, and was directly used for the next step reaction.
[0071] Step 2~4.
[0072] Except that the starting reactant was replaced by the product of step 1 of this example, the remaining steps were carried out according to steps 2-4 of example 1.
[0073] Finally, 110 g of off-white crystals were obtained, with a yield of 47% based on 2,4,5-trifluorophenylaceta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com