Application of USP 49 (ubiquitin-specific protease 49)
A specific, protease-based technology, applied in the field of biomedicine, can solve problems such as poor efficacy of pancreatic ductal carcinoma, and achieve significant technological progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
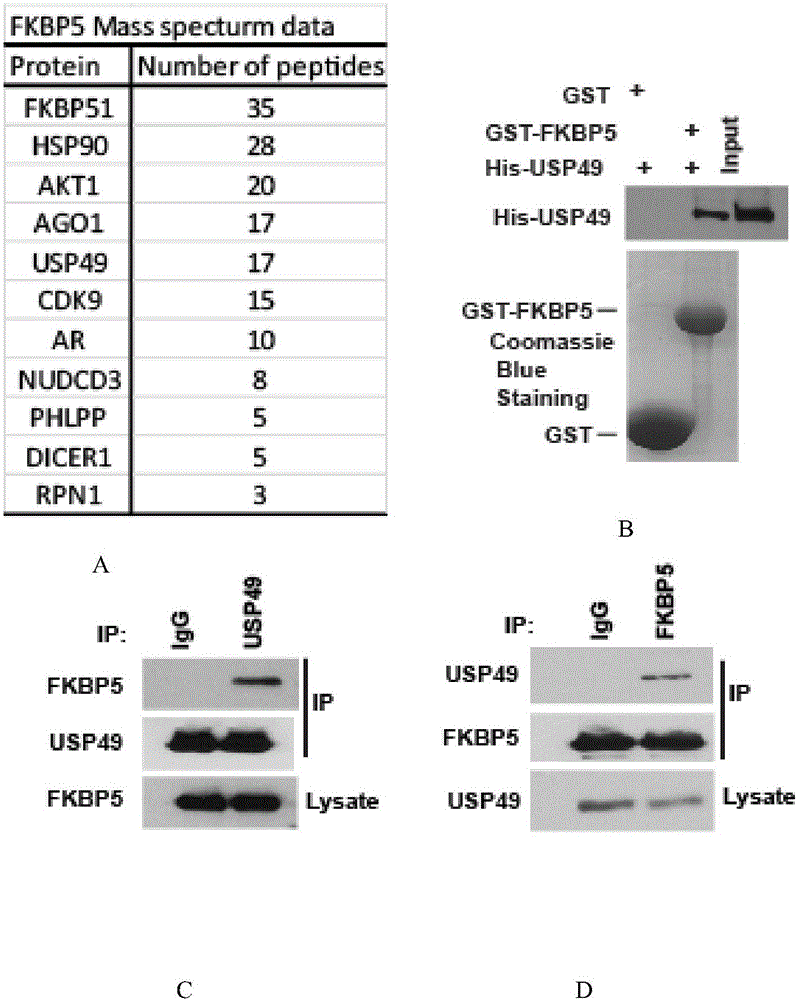
[0077] Example 1 Tandem affinity purification mass spectrometry analysis and identification of interacting proteins of FKBP5
[0078] Isolation and identification of protein complex components bound to FKBP5 using tandem affinity purification coupled with mass spectrometry (TAP-MS). Firstly, FKBP5 was constructed into the retroviral vector pMSCVpuro with Flag tag, and the correctness of the constructed new vector was verified by enzyme digestion identification and sequencing. The FKBP5 stable strain was constructed by virus infection, and after puro screening, the expression of the target protein FKBP5 was detected by western. The cell lines stably expressing FKBP5 were amplified in large quantities, and the tandem affinity purification was combined with mass spectrometry analysis to identify the interacting proteins that bind to FKBP5.
[0079] The result is as figure 1 As shown in A, tandem affinity purification combined with mass spectrometry successfully identified multi...
Embodiment 2
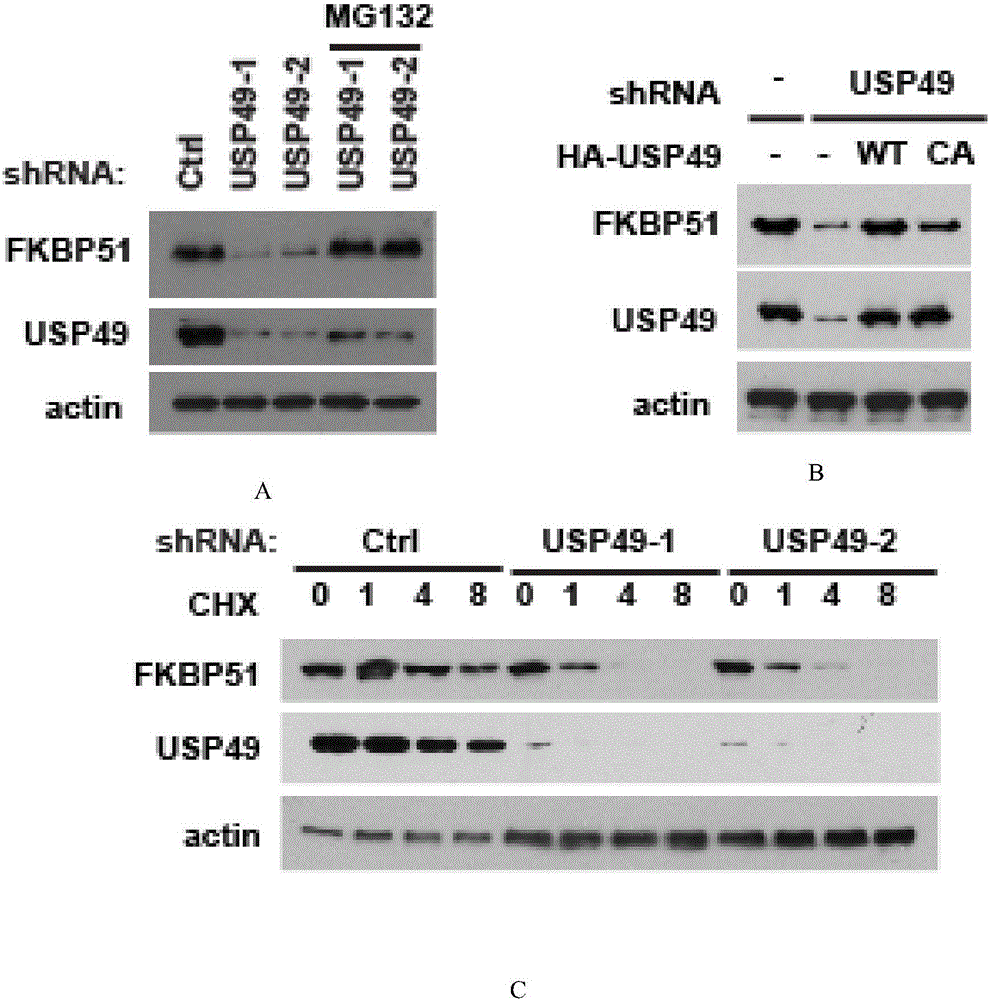
[0081] Example 2 USP49 can stabilize the protein level of FKBP5
[0082] The ubiquitin-proteasome pathway is an important protein degradation system in cells. Small ubiquitin molecules can mediate their degradation by binding to target proteins, and deubiquitinases (DUBs) are proteases that can reverse this process. Tritinylation of the tagged target protein stabilizes intracellular protein levels. figure 1 It has been confirmed that USP49 can interact with FKBP5. In this example, it is further tested whether USP49 can stabilize the protein level of FKBP5.
[0083] First, USP49 shRNA was used to establish a USP49-depleted stable cell line. Western results showed that, compared with the control, when the stable depleted USP49 protein was knocked out, the protein level of FKBP5 was also down-regulated ( figure 2 A). MG132 is a proteasome inhibitor that can prevent the degradation of target proteins through the ubiquitin-proteasome pathway. When MG132 was added to the USP49 st...
Embodiment 3
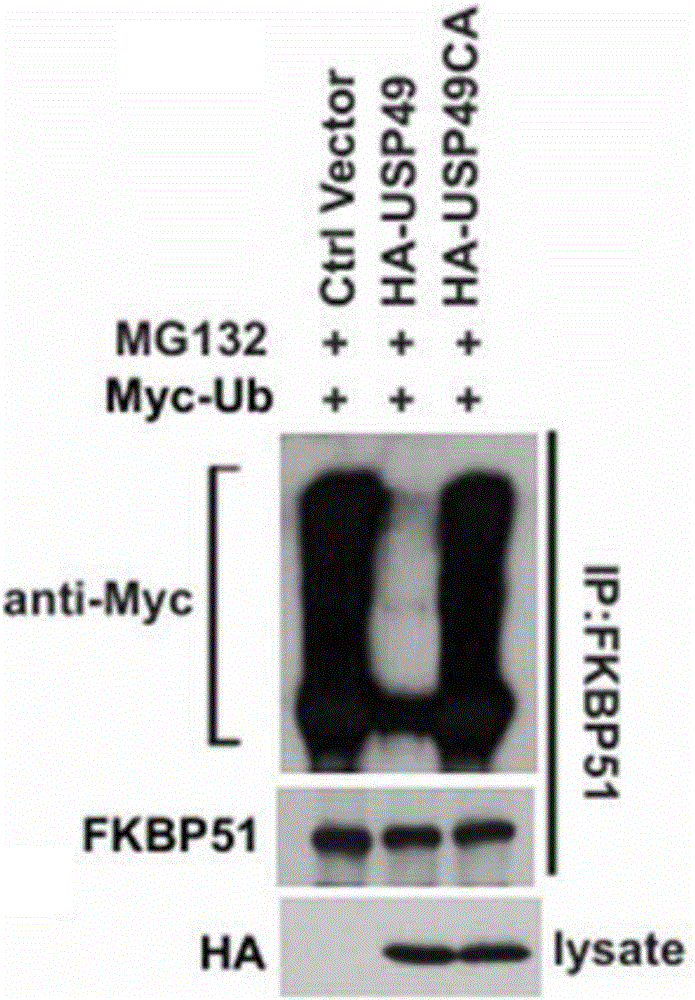
[0087] Example 3 USP49 regulates the ubiquitination level of FKBP5 protein
[0088] Co-transfect 293T cells with USP49 overexpression plasmid or USP49 catalytic inactivation mutant plasmid and ub plasmid, add MG132 for 4 hours after 48 hours, collect cells for ubiquitination experiment, the results are as follows image 3 As shown, USP49 wild type can obviously down-regulate the ubiquitination level of FKBP5, but USP49 catalytically inactive mutant loses this function. In addition, the ubiquitination level of FKBP5 was significantly increased when USP49 was stably depleted compared with the control group ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com