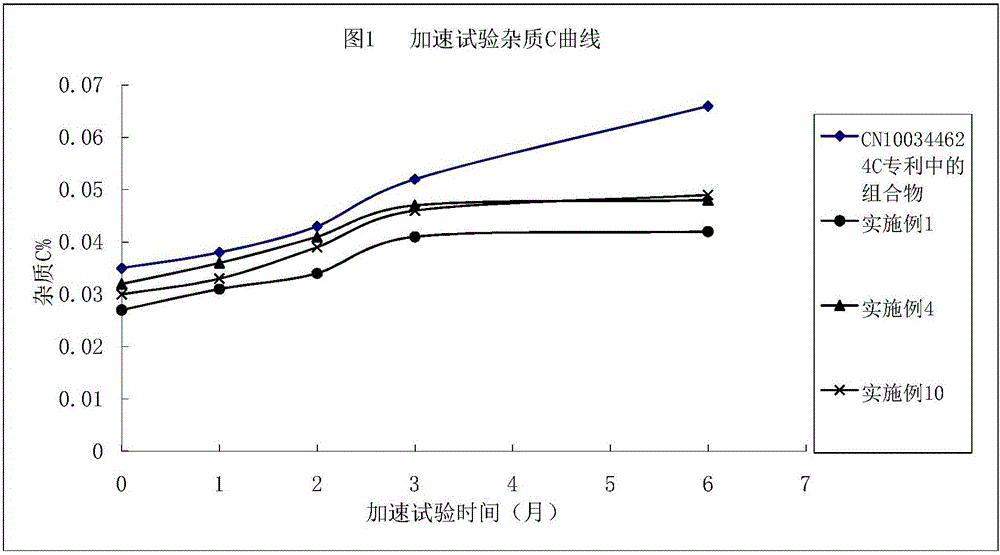
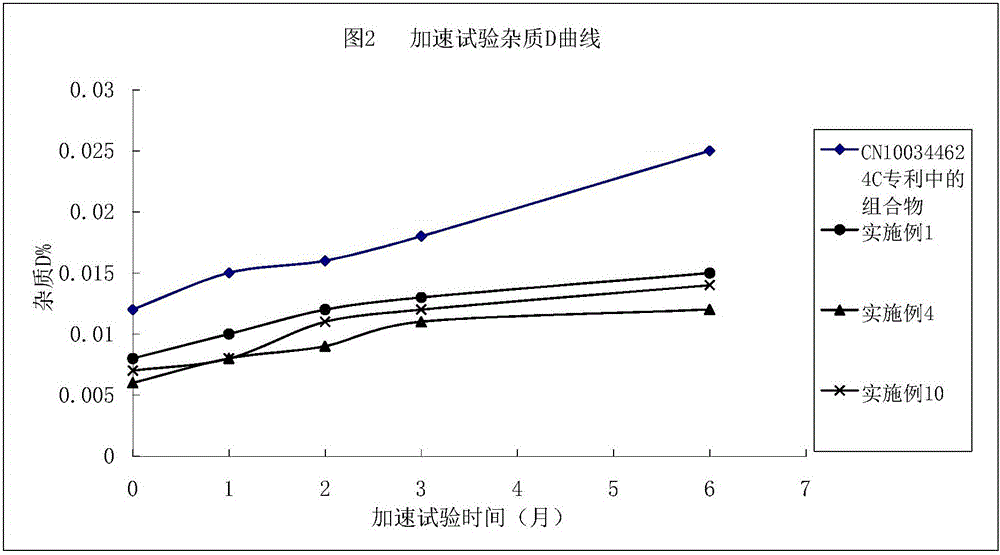
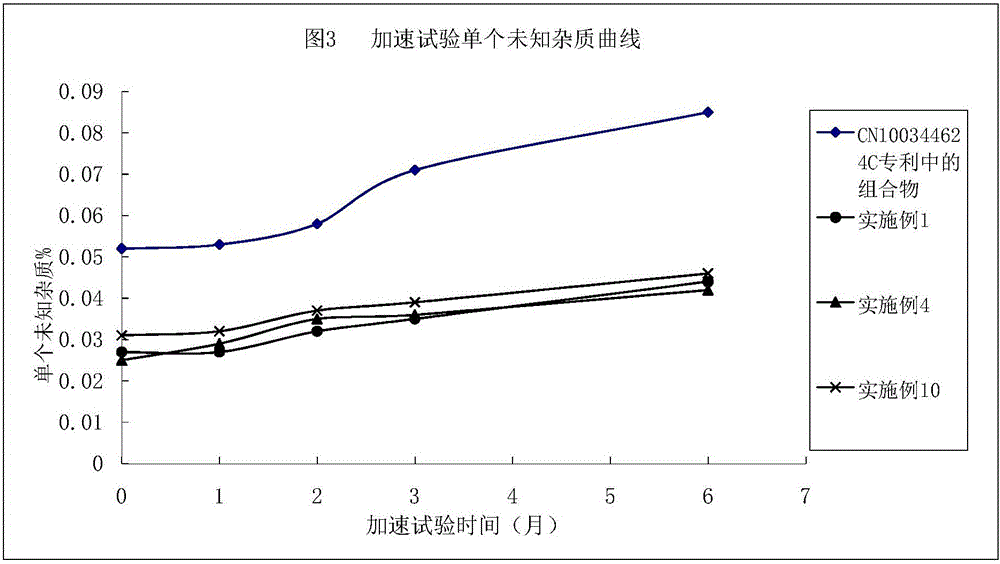
Argatroban composition and preparation method thereof
A technology of argatroban and its composition, applied in the field of chemical pharmacy, can solve the problems of slow cooling, large volume of liquid medicine, and inconvenient adjustment of pH value, etc., and achieve the effect of reducing cycle time, shortening working hours, and reducing steam consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]
[0087] 【Preparation】
[0088] A. Add sorbitol to 80% of the total amount of water for injection, stir to dissolve, add 0.1% (w / v) activated carbon for needles, stir for 10 minutes, filter to obtain solution A, and the pore size of the filter material is 0.8 μm.
[0089] B. Add an appropriate amount of ethanol to solution A to make a solution containing 75% (w / w) ethanol, add argatroban and stir to dissolve, add the remaining solution A, add water for injection to 95% of the total amount, Use 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value to 4.5-6.5, add water for injection to the total amount, stir and mix.
[0090] C. Take samples according to the law, measure the pH value and content of the medicinal liquid, and filter the medicinal liquid after the result is qualified. The filter material has a pore size of 0.22 μm, fills it with nitrogen gas and seals it. The inner packaging material is a 20ml brown ampoule, and...
Embodiment 2
[0092]
[0093] 【Preparation】
[0094] A. Add sorbitol to 70% of the total amount of water for injection, stir to dissolve, add 0.1% (w / v) activated carbon for needles, stir for 15 minutes, filter to obtain solution A, and the pore size of the filter material is 0.45 μm.
[0095] B. Add an appropriate amount of ethanol to solution A to make a solution containing 50% (w / w) ethanol, add argatroban and stir to dissolve, add the remaining solution A, add water for injection to 95% of the total, Use 0.1mol / L lactic acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value to 4.5-6.5, add water for injection to the total amount, and stir to mix.
[0096] C. Take samples according to the law, measure the pH value and content of the medicinal liquid, and filter the medicinal liquid after the result is qualified. The filter material has a pore size of 0.22 μm, fills it with nitrogen gas and seals it. The inner packaging material is a 20ml brown ampoule, and steriliz...
Embodiment 3
[0098]
[0099] 【Preparation】
[0100] A. Add sorbitol to 60% total amount of water for injection, stir to dissolve, add 0.1% (w / v) activated carbon for needles, stir for 20 minutes, filter to obtain solution A, and the filter material pore size is 0.22 μm.
[0101] B. Add an appropriate amount of ethanol to solution A to make a solution containing 50% (w / w) ethanol, add argatroban and stir to dissolve, add the remaining solution A, add water for injection to 95% of the total, Use 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value to 4.5-6.5, add water for injection to the total amount, stir and mix.
[0102] C. Take samples according to the law, measure the pH value and content of the medicinal liquid, and filter the medicinal liquid after the result is qualified. The filter material has a pore size of 0.22 μm, fills it with nitrogen gas and seals it. The inner packaging material is a 20ml brown ampoule, and sterilized by autoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com