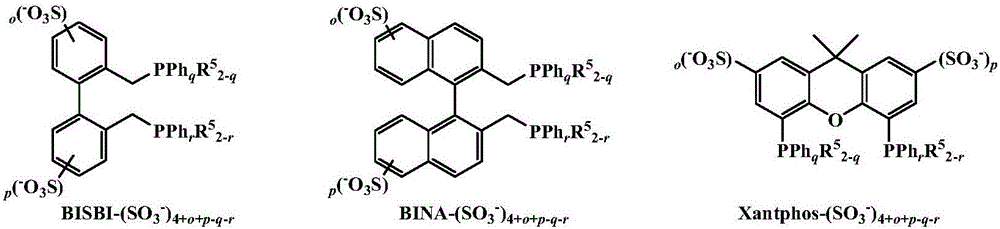
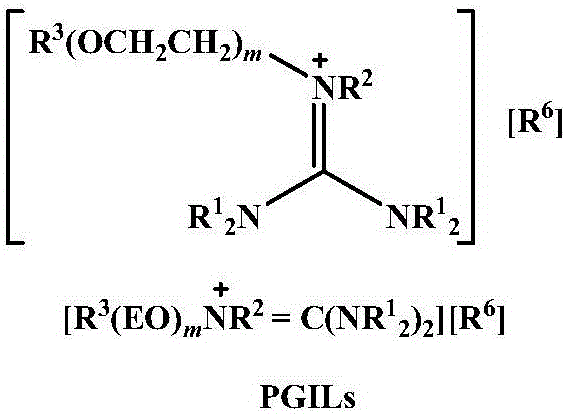
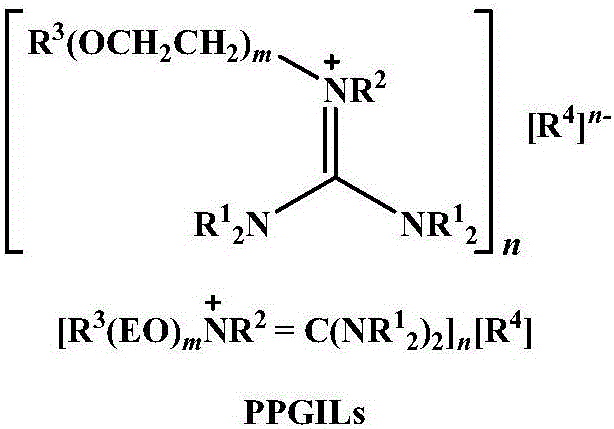Method for highly selective preparation of linear aldehyde by olefin two-phase hydroformylation based on phosphine functionalized polyether alkyl guanidine salt ionic liquid
A polyether alkyl guanidine, high-selectivity technology, applied to carbon monoxide reaction preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low catalytic activity and high dosage of ionic liquid , poor n-formaldehyde regioselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Rh(acac)(CO) 2 / BISBI-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0027] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , BISBI-(SO 3 Na) 2 , [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] and 1-octene, the ratio is: BISBI-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =1000:1 (molar ratio), [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde can be obtained through simple...
Embodiment 2
[0029] Rh(acac)(CO) 2 / BINA-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0030] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , BINA-(SO 3 Na) 2 , [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] and 1-octene in a ratio of: BINA-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =5000:1 (molar ratio), [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde can be obtained through simple tw...
Embodiment 3
[0032] Rh(acac)(CO) 2 / Xantphos-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0033] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , Xantphos-(SO 3 Na) 2 , [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] and 1-octene in a ratio of: Xantphos-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =1000:1 (molar ratio), [Ph(EO) 16 N + H=C(N(CH 3 ) 2 ) 2 ][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde can be obtained throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com