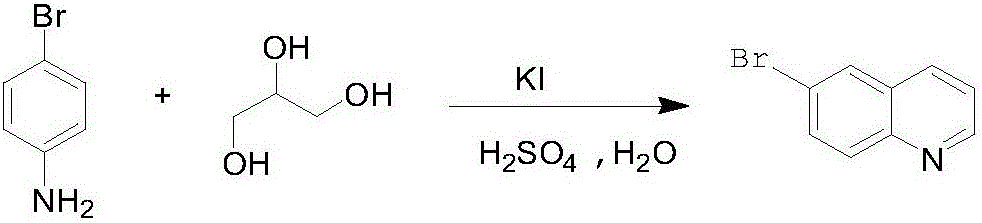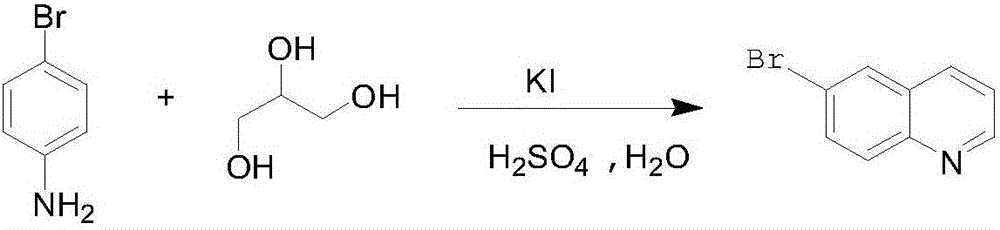Preparation method for 6-bromine quinoline
The technology of bromoquinoline and p-bromoaniline is applied in the field of preparation of 6-bromoquinoline, can solve the problems of high finished product, difficult recovery of solvent, complicated reaction steps and the like, and achieves the effects of simple and convenient operation, low price and low finished product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Put 0.1mol of p-bromoaniline, 0.43mol of sulfuric acid (concentration 80%), and 0.006mol of potassium iodide into the reaction bottle, heat up to 140-145°C, heat and stir until completely dissolved, and control the internal temperature of 140-145°C to the bottle Add 0.125 mol of glycerol dropwise. During the dropping process, water will be evaporated. After the dropping is completed, dehydrate at 140-145°C for 3 hours to check whether there are any raw materials remaining. The product has a purity of 85.4%, and quinoline is 10.2% (GC);
[0020] (2) Drop the reaction solution from the previous step into water, and adjust the pH to 6-7 with 0.35 mol of ammonia water;
[0021] (3) Add 200ml toluene to the upward step reaction solution for extraction and layering, then extract the aqueous phase twice with 200ml toluene, extract toluene and concentrate to obtain black 6-bromoquinoline crude product, purity 92%, quinoline 4% (HPLC). Control vacuum ≤ 15mmHg, collect 150~15...
Embodiment 2
[0023] (1) Put 0.1mol p-bromoaniline, 0.43mol sulfuric acid (concentration 70%), and 0.006mol potassium iodide into the reaction bottle, heat up to 140-145°C, heat and stir until they are completely dissolved, and control the internal temperature of 140-145°C to the bottle Add 0.125 mol of glycerol dropwise. During the dropwise addition, water will be evaporated. After the dropwise addition, dehydrate at 140-145°C for 3 hours to detect 1.7% of raw material residue, product purity 79.4%, and quinoline 12.8% (GC);
[0024] (2) Drop the reaction solution from the previous step into water, and adjust the pH to 6-7 with 0.35 mol of ammonia water;
[0025] (3) Add 200ml of toluene to the upward step reaction solution for extraction and layering, then extract the water phase twice with 200ml of toluene, extract the toluene and concentrate to obtain black 6-bromoquinoline crude product, purity 87.3%, quinoline 7.5% (HPLC). Control vacuum ≤ 15mmHg, collect 150~155 ℃ distillate, the 6-b...
Embodiment 3
[0027] (1) Put 0.1mol p-bromoaniline, 0.43mol sulfuric acid (concentration 90%), and 0.006mol potassium iodide into the reaction bottle, heat up to 140-145°C, heat and stir until they are completely dissolved, and control the internal temperature of 140-145°C to the bottle Add 0.125 mol of glycerol dropwise. During the dropwise addition, water will be evaporated. After the dropwise addition, dehydrate at 140-145°C for 3 hours to detect 0% of the raw material residue, product purity 75.4%, and quinoline 19.8% (GC);
[0028] (2) Drop the reaction solution from the previous step into water, and adjust the pH to 6-7 with 0.35 mol of ammonia water;
[0029] (3) Add 200ml of toluene to the upward step reaction solution for extraction and layering, and the aqueous phase is extracted twice with 200ml of toluene, and the extraction toluene is combined and concentrated to obtain black 6-bromoquinoline crude product with a purity of 84%, quinoline 10.9% (HPLC). Control vacuum ≤ 15mmHg, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com