Chiral pyridine biimidazole ligand transition metal complex catalyst and preparation method thereof
A technology of pyridine bis-imidazole and transition metal, which is applied in the field of chiral pyridine bis-imidazole ligand transition metal complex catalyst and its preparation, can solve the problems of expensive ligands and difficult synthesis, and achieve cheap and easy-to-obtain raw materials. The effect of simple synthesis operation and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
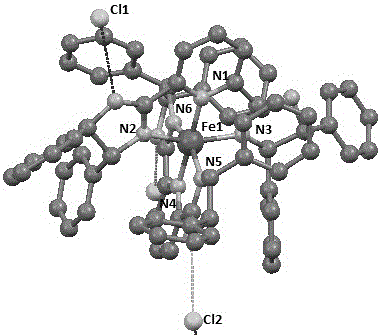
[0036] Embodiment 1: prepare chiral pyridine bis-imidazole ligand-iron complex 1a of the present invention, its structural formula is:
[0037]
[0038] In the glove box, the FeCl 2 (126.8 mg, 1.0 mmol, 1.0 equiv) and THF (50 mL) were added to a 100 mL schlenk tube, and then a THF solution (10 mL) of chiral pyridine bis-imidazole ligand (1.04 g, 2.0 mmol, 2.0 equiv) was slowly added The above solution was added dropwise, and at the moment of addition, the color of the reaction solution rapidly changed to purple-black, and a purple-black solid was precipitated. After the reaction was stirred at room temperature for 24 h, it was filtered, washed with THF and ether, and the solvent was drained to obtain a purple-black powder (1.02 g, 87%). Then put the above powder (15 mg) into a sample bottle with a volume of 4 mL, dissolve it in DMSO (1 mL), transfer the above sample bottle to a sample bottle with a volume of 18 mL containing ether solvent, and the ether solvent cannot cov...
Embodiment 2
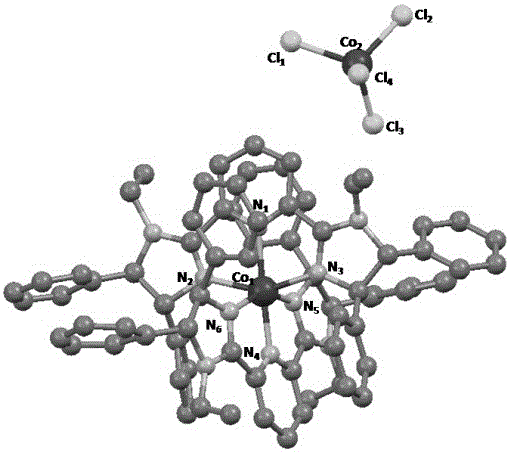
[0039] Embodiment 2: Preparation of chiral pyridine bis-imidazole ligand-iron complex [(NNN) of the present invention 2 Fe] 2+ FeCl 4 2- (complex 1b), its structural formula is:
[0040]
[0041] In the glove box, the FeCl 2 (126.8 mg, 1.0 mmol, 1.0 equiv) and THF (50 mL) were added to a 100 mL schlenk tube, and then a THF solution (10 mL) of chiral pyridine bis-imidazole ligand (575.7 mg, 1.0 mmol, 1.0 equiv) was slowly added The above solution was added dropwise, and at the moment of addition, the color of the reaction solution rapidly changed to purple-black, and a purple-black solid was precipitated. After the reaction was stirred at room temperature for 24 h, it was filtered, washed with THF and ether, and the solvent was drained to obtain a purple-black powder (583.1 mg, 83%). Anal. Calcd for C 78 h 74 Cl 4 Fe 2 N 10 : C, 66.68; H, 5.31; N, 9.97. Found: C, 67.06; H, 5.68; N, 10.26.
Embodiment 3
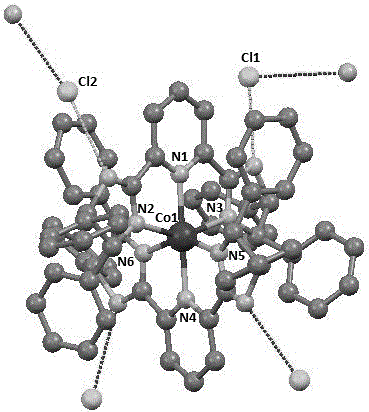
[0042] Embodiment 3: Preparation of chiral pyridine bis-imidazole ligand-iron complex [(NNN) of the present invention 2 Fe] 2+ Cl 2 2- (complex 1c), its structural formula is:
[0043]
[0044] In the glove box, the FeCl 2 (126.8 mg, 1.0 mmol, 1.0 equiv) and THF (50 mL) were added to a 100 mL schlenk tube, and then a THF solution (10 mL) of chiral pyridine bis-imidazole ligand (1.11 g, 2.0 mmol, 2.0 equiv) was slowly added The above solution was added dropwise, the color of the reaction solution rapidly changed to purple-black, and a purple-black solid was precipitated. After the reaction was stirred at room temperature for 24 h, it was filtered, washed with THF and ether, and the solvent was drained to obtain a purple-black powder (1.00 g, 81%). Anal. Calcd for C 70 h 56 Cl 4 FeN 10 : C, 68.08; H, 4.57; N, 11.54. Found: C, 68.53; H, 4.91; N, 11.70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com