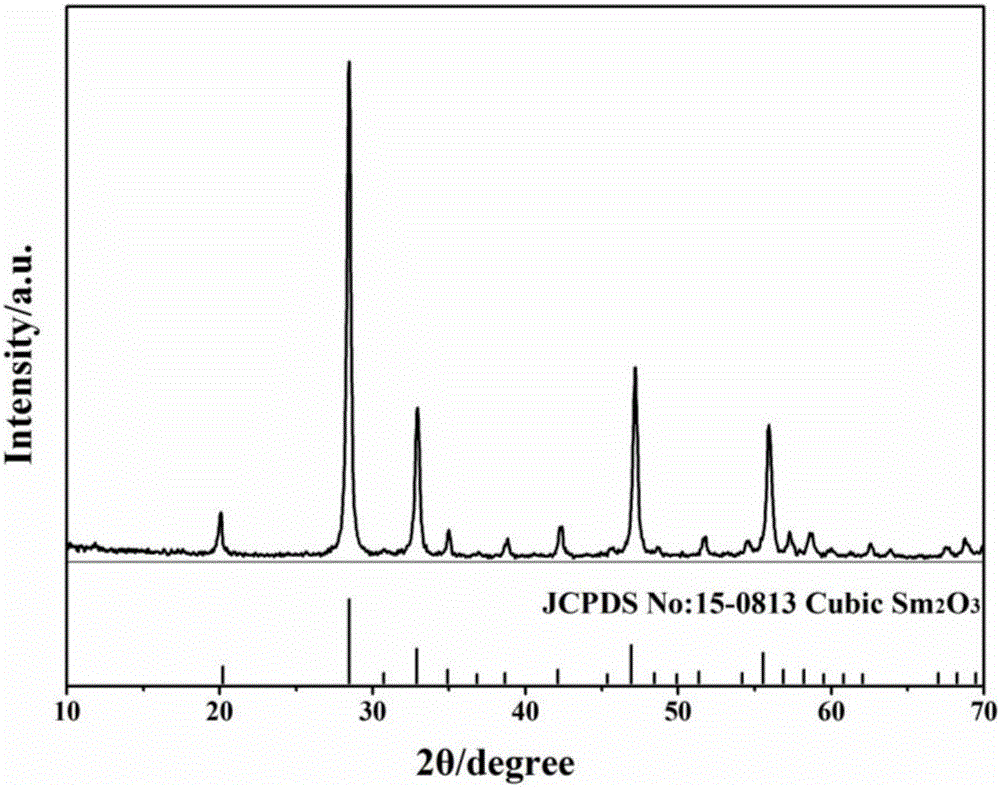
a sm 2 o 3 Preparation method of nanocrystal
A nanocrystal and reaction technology, applied in chemical instruments and methods, rare earth metal oxides/hydroxides, rare earth metal compounds, etc., can solve the problem of high heat treatment temperature, achieve high purity, low production cost, and good industrialization prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the present invention comprises the following steps:
[0028] 1) A certain amount of analytically pure Sm(NO 3 ) 3 ·6H 2 O was dissolved in appropriate amount of distilled water to make Sm 3+ The concentration is 0.02~0.06mol·L -1 Samarium nitrate solution;
[0029] 2) A certain amount of analytically pure CO(NH 2 ) 2 Dissolved in appropriate amount of distilled water for alkali source to make concentration 0.01~0.03mol L -1 urea solution;
[0030] 3) Mix equal volumes of samarium nitrate solution and urea solution under magnetic stirring to form a reaction precursor, because the two are mixed in equal volumes, therefore, Sm(NO 3 ) 3 and CO(NH 2 ) 2 The molar ratio is (0.02~0.06):(0.01~0.03);
[0031]4) Pour the above-mentioned reaction precursor solution into an ordinary hydrothermal kettle, control the filling degree of the reaction precursor solution in the hydrothermal kettle to be 60% to 80%, seal the hydrothermal kettle, put i...
Embodiment 1
[0036] 1) A certain amount of analytically pure Sm(NO 3 ) 3 ·6H 2 O was dissolved in appropriate amount of distilled water to make Sm 3+ The concentration is 0.02mol·L -1 solution A;
[0037] 2) A certain amount of analytically pure CO(NH 2 ) 2 Dissolved in an appropriate amount of distilled water to make a concentration of 0.01mol L -1 Solution B of
[0038] 3) Mix equal volumes of solution A and solution B under magnetic stirring to form a reaction precursor;
[0039] 4) Pour the above-mentioned reaction precursor solution into an ordinary hydrothermal kettle, control the filling degree at 80%, seal the hydrothermal kettle, put it into an electric drying oven, and react at 160°C for 16 hours, and naturally cool to room temperature after the reaction;
[0040] 5) The product was sequentially washed with distilled water and absolute ethanol for 4 times, and dried in an electric oven at 60° C. for 4 hours to obtain the precursor.
[0041] 6) Calcinate the obtained prec...
Embodiment 2
[0043] 1) A certain amount of analytically pure Sm(NO 3 ) 3 ·6H 2 O was dissolved in appropriate amount of distilled water to make Sm 3+ The concentration is 0.04mol L -1 solution A;
[0044] 2) A certain amount of analytically pure CO(NH 2 ) 2 Dissolved in an appropriate amount of distilled water to make a concentration of 0.02mol L -1 Solution B of
[0045] 3) Mix equal volumes of solution A and solution B under magnetic stirring to form a reaction precursor;
[0046] 4) Pour the above-mentioned reaction precursor solution into an ordinary hydrothermal kettle, control the filling degree at 70%, seal the hydrothermal kettle, put it into an electric drying oven, and react at 140°C for 18 hours, and naturally cool to room temperature after the reaction;
[0047] 5) The hydrothermal product was sequentially washed with distilled water and absolute ethanol four times, and dried in an electric drying oven at 70° C. for 3 hours to obtain the precursor.
[0048] 6) Calcinat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com