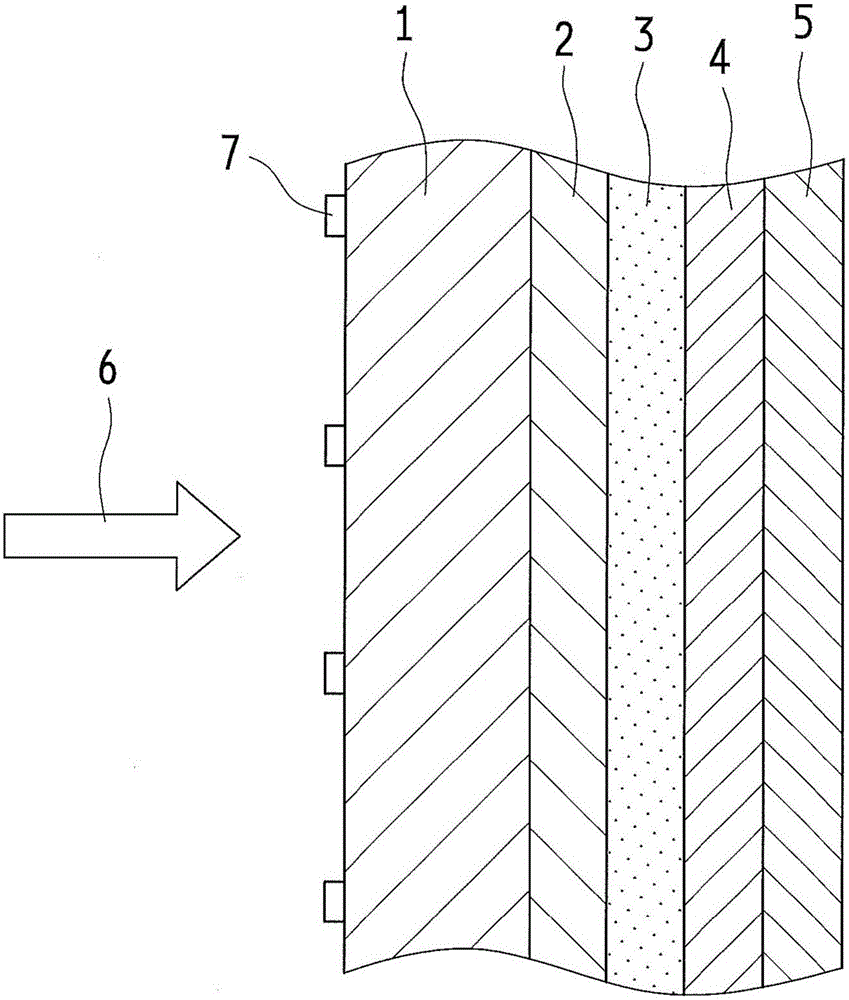
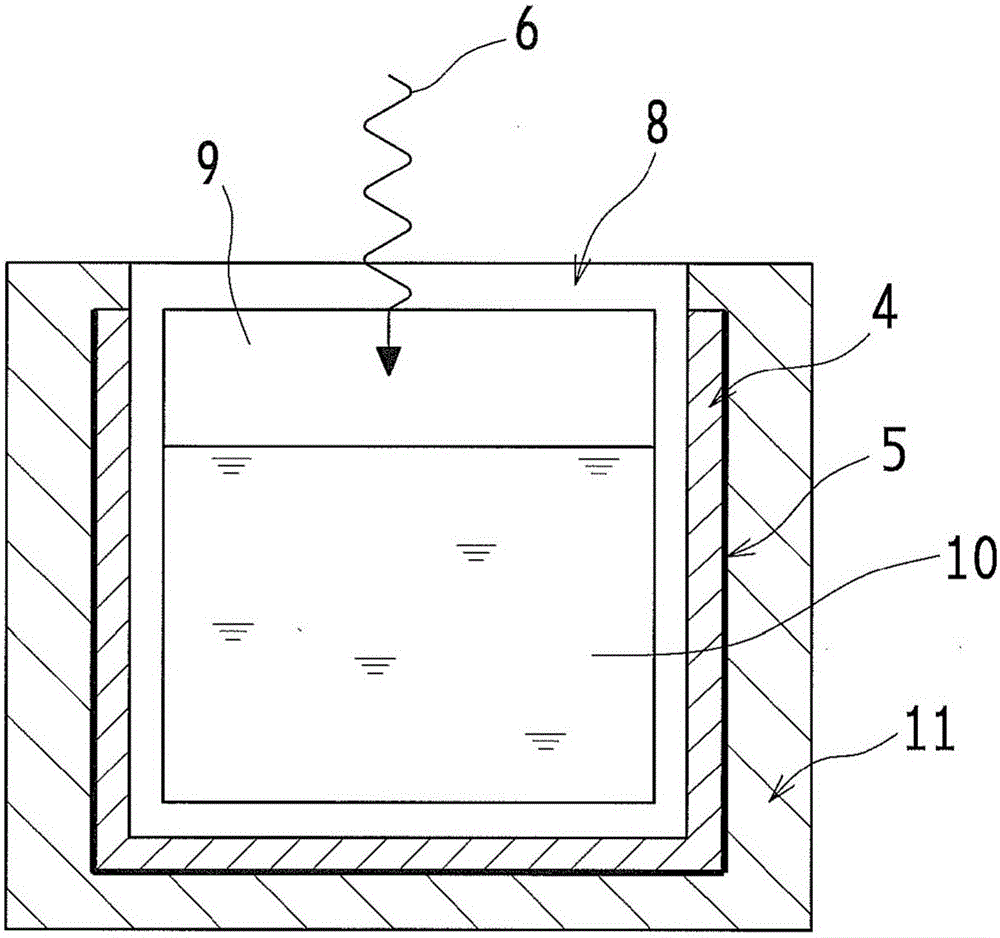
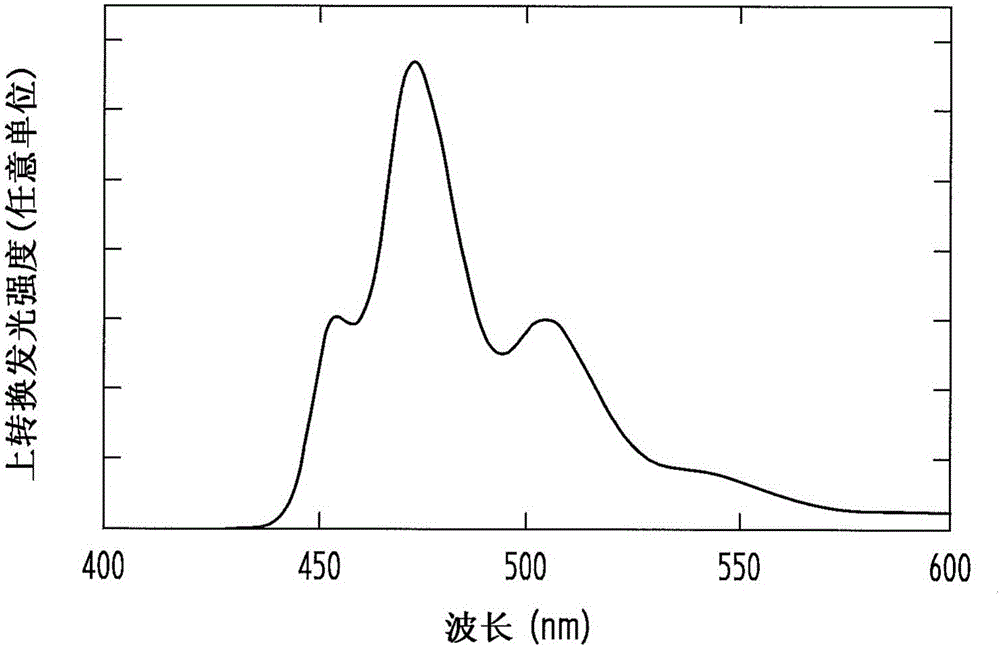
Optical wavelength conversion element containing ionic liquid, and article equipped with said optical wavelength conversion element
A light wavelength conversion, ionic liquid technology, applied in optical components, electrical components, semiconductor devices, etc., can solve the problems of up-conversion light intensity reduction, flammable media, volatility, low fluidity, and inability to use resin materials, etc. Achieve the effect of good time stability and high optical wavelength conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0193] Next, the present invention will be described in more detail by way of examples. It should be noted that the present invention is not limited by the following examples. The ultrapure water used in the preparation example of the following ionic liquid (C) is as follows.
[0194] (manufacture of ultrapure water)
[0195] In the production example of the following ionic liquid (C), ultrapure water produced by an ultrapure water production device (manufacturer: Merck, model: Direct-Q (registered trademark) UV3) was used as ultrapure water .
Synthetic example 1
[0197] Using the method described in Non-Patent Document 6, the organic photosensitive molecule (A) (2-iodo-1,3,5,7-tetramethyl-8-phenyl-4,4- Difluoroboradiazaindacene).
[0198]
[0199] The obtained compound was identified by the following NMR spectrum.
[0200] 1 H NMR (400MHz, CDCl 3 ): δ7.51-7.48(m,3H),7.27-7.25(m,2H),6,04(s,1H),2.63(s,3H),2.57(s,3H),1.38(s,6H )
[0201] 13 C NMR (100MHz, CDCl 3 ): δ157.9, 154.7, 145.3, 143.4, 141.7, 135.0, 132.0, 131.1, 129.8, 129.5, 129.4, 128.0, 122.5, 84.4, 16.8, 16.0, 14.9, 14.7
Synthetic example 2
[0203] Utilizing the method described in Non-Patent Document 6, an organic photosensitive molecule (A) represented by the following formula (2,6-diiodo-1,3,5,7-tetramethyl-8-phenyl-4 ,4-Difluoroboradiazepinedacene).
[0204]
[0205] The obtained compound was identified by the following NMR spectrum.
[0206] 1 H NMR (400MHz, CDCl 3 ): δ7.54-7.51(m,3H),7.26-7.24(m,2H),2.65(s,6H),1.38(s,6H)
[0207] 13 C NMR (100MHz, CDCl 3 ): δ156.9, 145.5, 141.5, 134.4, 129.7, 129.6, 127.9, 85.8, 17.1, 16.2
[0208] (Preparation example 1 of ionic liquid (C))
[0209] In a small glass vial, put 1-propyl-2,3-dimethylimidazole as a water-immiscible ionic liquid A commercially available product (manufacturer: Ionic Liquids Technologies, Inc.) of bis(trifluoromethanesulfonyl)imide (CAS No.: 169051-76-7; hereinafter referred to as "ionic liquid #1") was supplied to the ion Add 9 times the volume of ultrapure water to the commercially available liquid #1, stir it with a conventional mag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com