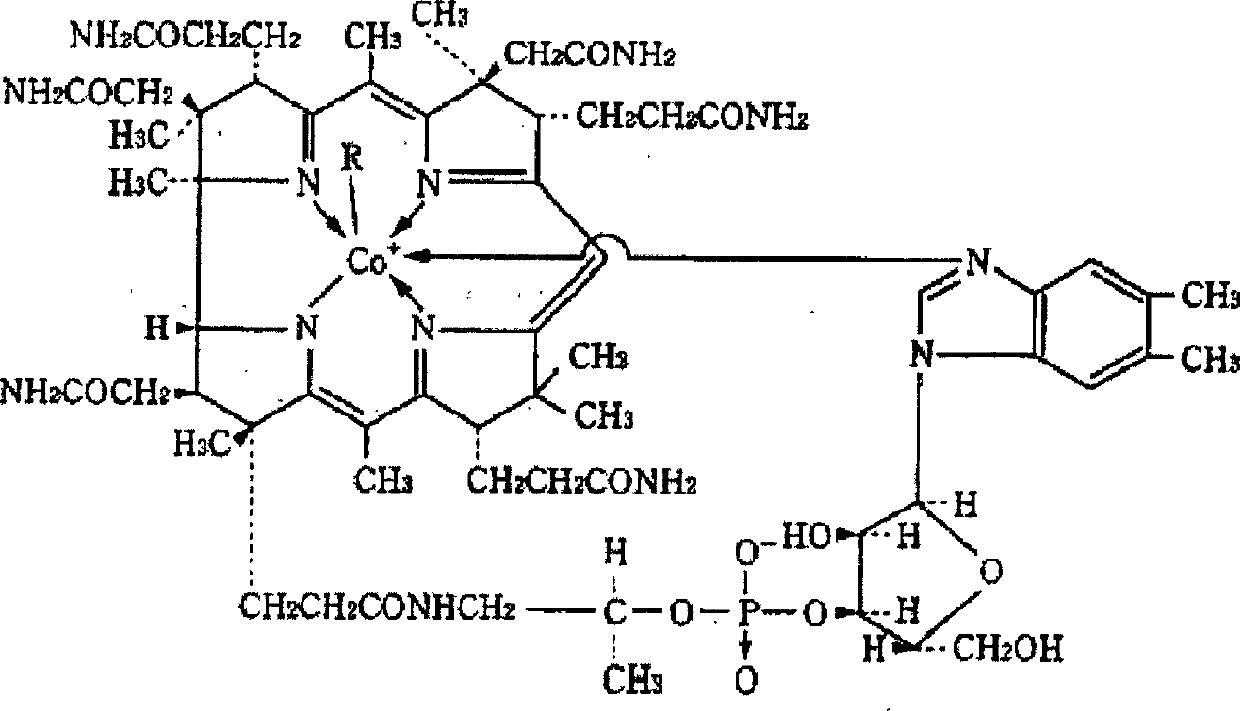
Freeze-dried preparation containing methylcobalamin and process for producing the same
A technology for methyl vitamins and freeze-dried preparations, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and freeze-dried delivery, etc. Storage stability and other issues, to achieve the effect of excellent stability over time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
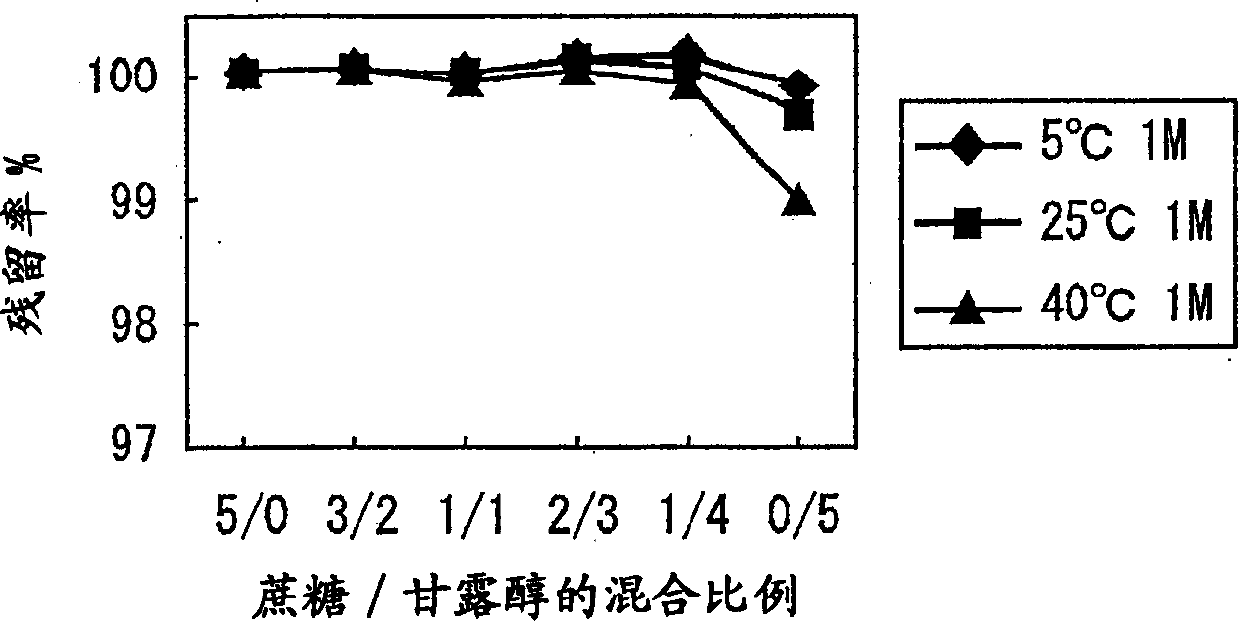
[0091] As one embodiment of the preparation method of the freeze-dried preparation of the present invention, based on the mixing ratio described later, an excipient composed of a mixture of sucrose and mannitol and vitamin B methyl 12 A raw material solution was prepared together, and it was prepared by the following freeze-drying method.
[0092] 0.5% (w / v) methyl vitamin B 12 (trade name Methycobal: manufactured by Eisai Co., Ltd.) and 2.5% (w / v) excipient mixture were mixed to prepare a raw material solution, which was sterile-filtered and aseptically dispensed into each vial in an accurate amount for lyophilization. . After freeze-drying, stopper completely, make the vitamin B containing methyl of the present invention 12 freeze-dried preparations.
[0093] Specifically, put the amount according to the mixing ratio of sucrose and mannitol shown in Figure 1 into a 100ml beaker, add 80ml of distilled water for injection, and stir it with a magnetic stirrer to dissolve it....
manufacture example 2
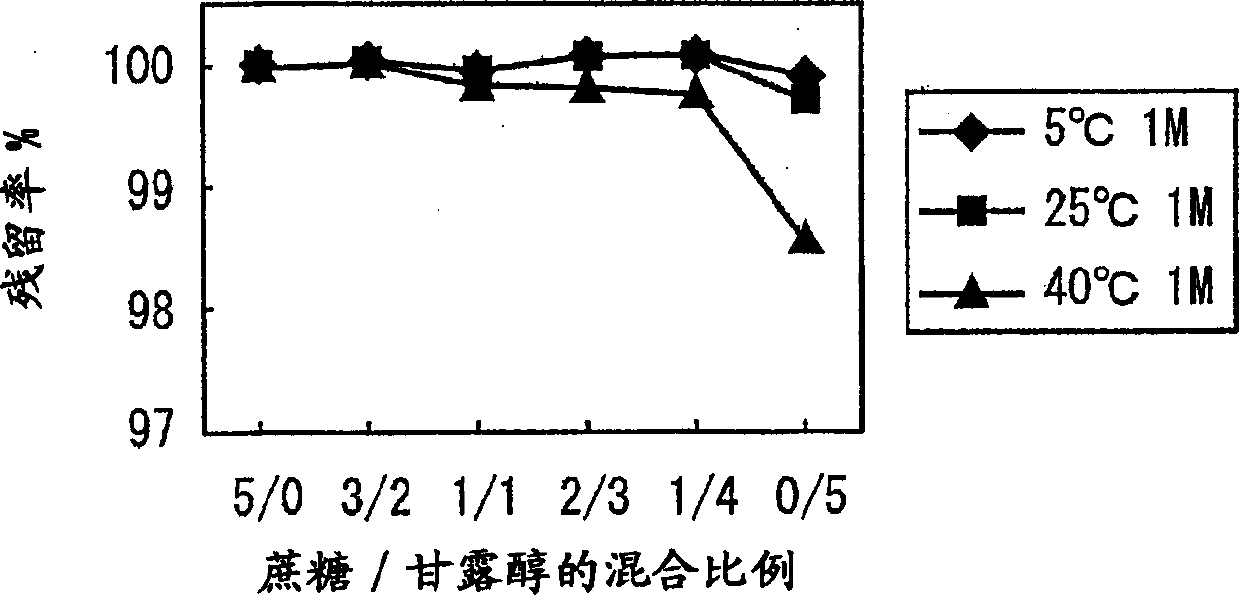
[0097] 0.5% (w / v) methyl vitamin B 12 (trade name Methycobal: manufactured by Eisai Co., Ltd.) and 5.0% (w / v) excipient mixture were mixed to prepare a raw material solution, which was subjected to sterile filtration and aseptically dispensed into vials in exact amounts for lyophilization. . After freeze-drying, stopper completely, make the vitamin B containing methyl of the present invention 12 freeze-dried preparations.
[0098] Specifically, put the amount according to the mixing ratio of sucrose and mannitol shown in Figure 2 into a 100ml beaker, add 80ml of distilled water for injection, and stir it with a magnetic stirrer to dissolve it. After confirming that the above excipients are fully dissolved, add 500 mg of methyl vitamin B 12 , Stir, after confirming that it is fully dissolved, use a 100ml volumetric flask to make up to volume, add distilled water to make the total solution volume 100ml.
[0099] Then, the above solution was divided into 18ml vials, 5ml per b...
manufacture example 3
[0102] Next, the mixture of lactose and mannitol as an excipient was based on the mixing ratio shown in Fig. 12 Raw material solutions were prepared together, and lyophilized preparations (Examples 11 and 12) were prepared under the same conditions as in Production Example 1.
[0103] In addition, as Comparative Example 3, a lyophilized preparation containing only mannitol shown in FIG. 3 as an excipient was prepared by the same method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| light receiving slit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com