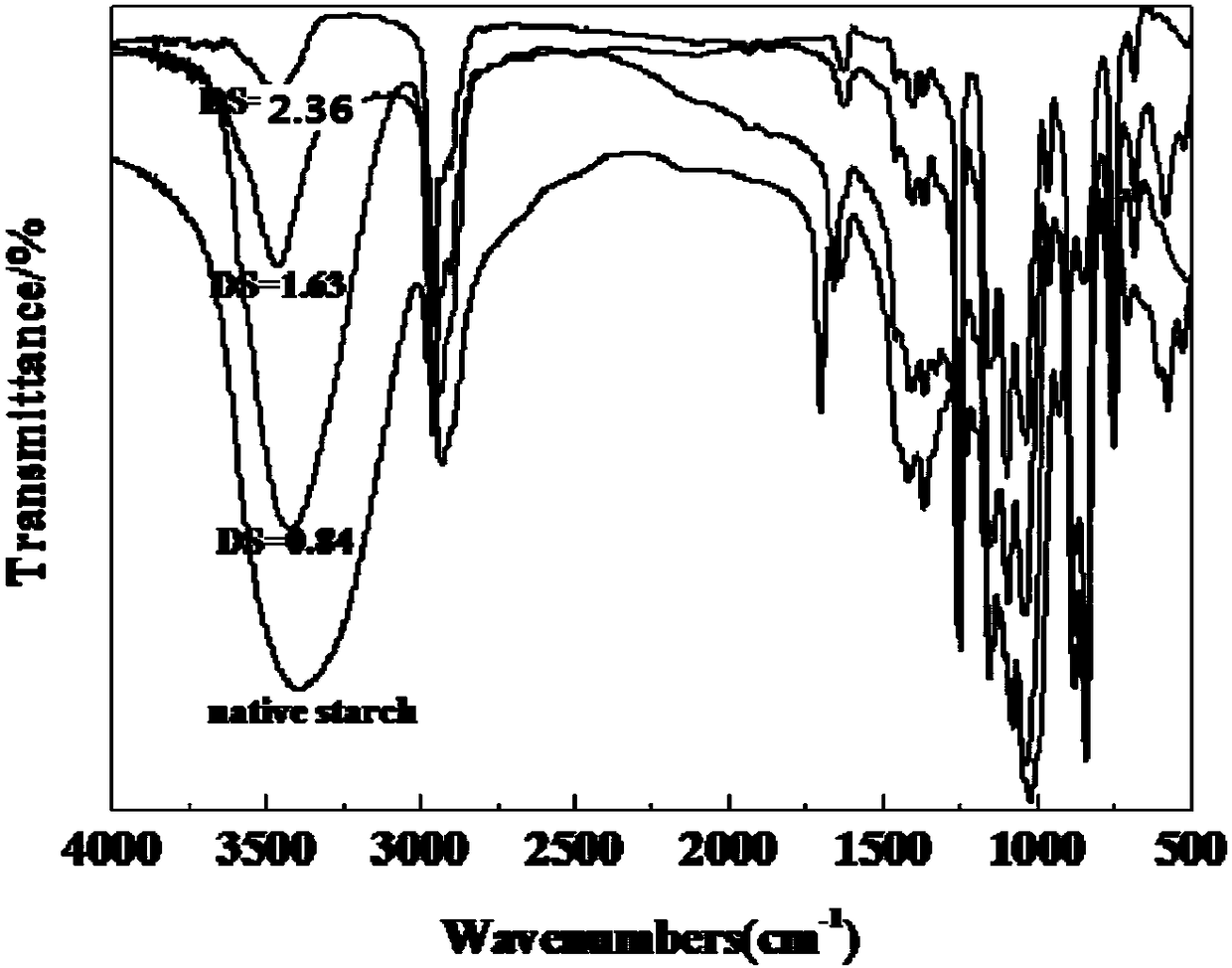
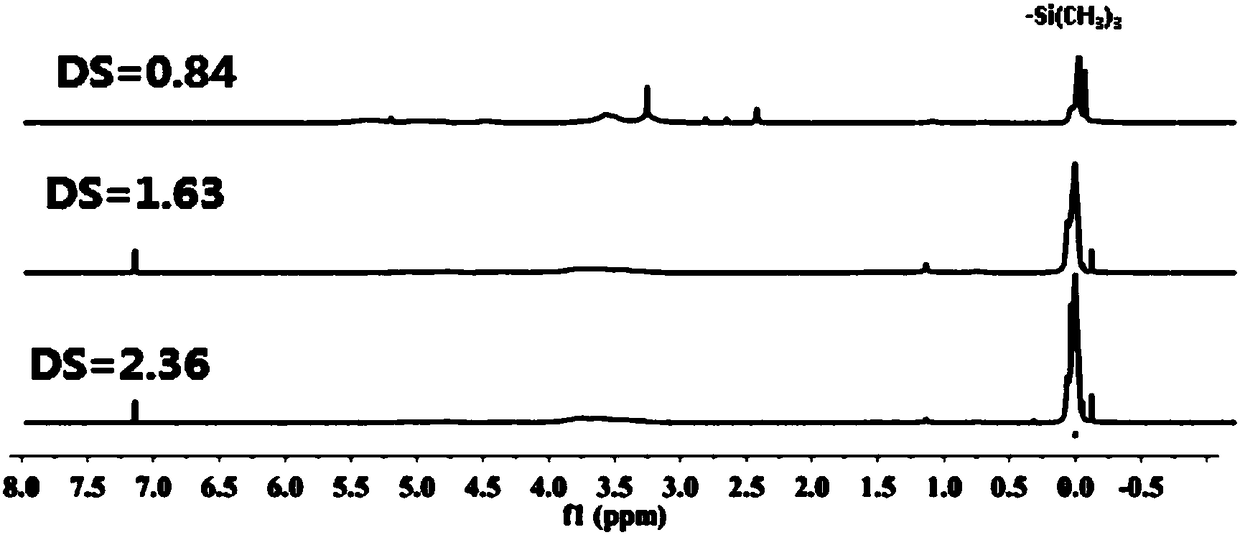
A kind of preparation method of trimethylsilyl starch ether
A technology of trimethylsilyl and starch ether, which is applied in the field of preparation of trimethylsilyl starch ether, and achieves the effects of simple operation process, mild reaction conditions and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Get the waxy corn starch of 10g, add the DMF of 100ml as solvent, according to n(Cu(NO 3 ) 2 ·3H 2 O):n(HMDS)=2.1:100, add Cu(NO 3 ) 2 ·3H 2 O as a catalyst, 50W ultrasonic dispersion into a homogeneous system, at 50°C, add HMDS dropwise according to n(HMDS):n(AGU)=3:1, stir magnetically, sample the gas phase every half an hour to analyze the HMDS content, gas phase The end point of the reaction was determined by chromatography. After 7 hours of reaction, the conversion rate of HMDS was 100%. After the reaction, the product was cooled to room temperature, the upper solvent was poured out, and ethanol and deionized water were poured into the lower product to precipitate the product, then suction filtered, and vacuum-dried at 90°C to obtain the crude product of trimethylsilyl starch ether . Grinding the crude product of trimethylsilyl starch, dissolving it in acetone, centrifuging to obtain a supernatant, concentrating the supernatant, adding a large amount of deion...
Embodiment 2
[0033] Get the waxy corn starch of 10g, add the DMF of 100ml as solvent, according to n(Cu(NO 3 ) 2 ·3H 2 O): n(HMDS)=2.1% add Cu(NO 3 ) 2 ·3H 2O as a catalyst, 50W ultrasonic dispersion into a homogeneous system, at 80 ° C, according to n (HMDS) and n (AGU) = 3:1, add HMDS dropwise, magnetic stirring, sampling the gas phase every half an hour to analyze the HMDS content, gas phase The end point of the reaction was determined by chromatography. After 2 hours of reaction, the conversion rate of HMDS was 100%. After the reaction, the product was cooled to room temperature, the upper layer solvent was poured out, and a large amount of ethanol and deionized water were poured into the lower layer product to precipitate the product, which was then filtered by suction and vacuum-dried at 90°C to obtain crude trimethylsilyl starch ether. product. After grinding the crude product of trimethylsilyl starch, dissolve it in acetone, centrifuge to obtain a supernatant, concentrate the...
Embodiment 3~4
[0038] Add HMDS dropwise according to the ratio of n(HMDS) and n(AGU)=1:1 and 2:1, other conditions are consistent with Example 1, and the products with degrees of substitution of 0.84 and 1.63 are obtained, and the yields are 84% and 81.5% respectively. %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com