Preparation method and application of near infrared GSH (glutathione) fluorescent probe
A fluorescent probe and near-infrared technology, applied in the field of fluorescent probes, can solve the problems of limited application and achieve rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of fluorescent probes
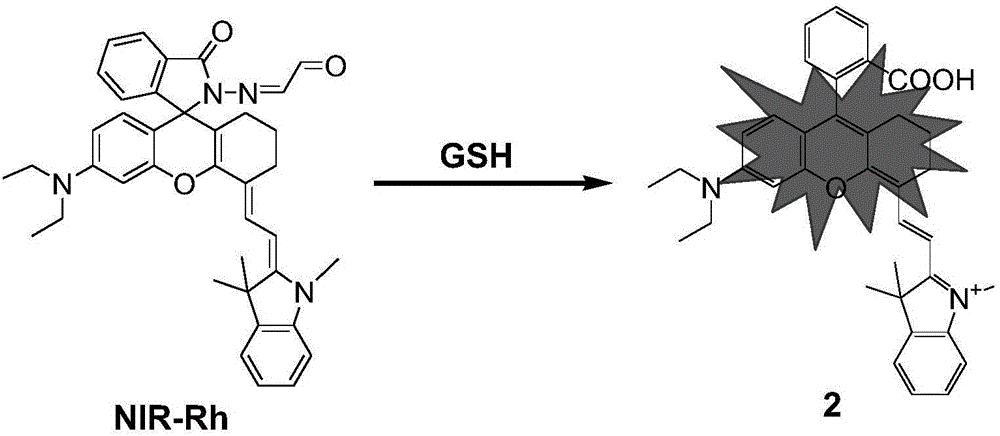
[0033] Synthesis of Compound 1: Cyclohexanone (6.4mmol, 0.66mL) was added dropwise to concentrated sulfuric acid (7.0mL) cooled to 0°C, followed by the addition of 2-((4-diethylamino)-2-hydroxybenzene Formyl)benzoic acid (3.2mmol, 1.003g), magnetically stirred and refluxed for 1.5 hours, and the reaction was stopped; after the reaction mixture was cooled to room temperature, the reaction mixture was poured into a beaker containing 50g of ice water under stirring, and then 0.7mL of 70% perchloric acid immediately produced a large amount of red precipitate. After suction filtration and drying, a red solid compound 1 was obtained, which was directly used in the next reaction without further purification. 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=8.0Hz, 1H), 7.78(t, J=7.2Hz 1H), 7.69(t, J=7.6Hz 1H), 7.22(d, J=7.5Hz, 1H), 7.06 -7.13(q,2H),6.89(s,1H),3.61-3.66(q,4H),3.07-3.18(m,2H),2.29-2.31(m,2H),1.99(s,2H),1.79 (s,2H),1.34(t,J=6.6Hz,6H).MS(TO...
Embodiment 2
[0038] Solution preparation of fluorescent probe interacting with GSH
[0039] A certain amount of fluorescent probe was dissolved in H 2 In the mixed solution of O / EtOH (9:1, v / v), the concentration obtained is 1.0×10 -4 mol L -1 Probe stock solution. Dissolve a certain amount of GSH in water, transfer it to a 500mL volumetric flask, add water to the mark, and obtain a concentration of 1.0×10 -2 mol L -1 GSH. will be 1.0×10 -2 mol L -1 The GSH solution was gradually diluted with water to obtain 1.0 x 10 -3 -1.0×10 -8 mol L -1GSH aqueous solution. Add 1.0mL of the probe stock solution and 1.0mL of GSH aqueous solution into a 10mL volumetric flask, and dilute to the volume with buffer solution to obtain a concentration of 1.0×10 -5 mol L -1 fluorescent probe and 1.0 x 10 -3 -1.0×10 -8 mol L -1 GSH mixed with the solution to be tested.
Embodiment 3
[0041] Measurement of Fluorescence Spectrum of Interaction of Fluorescent Probe with GSH
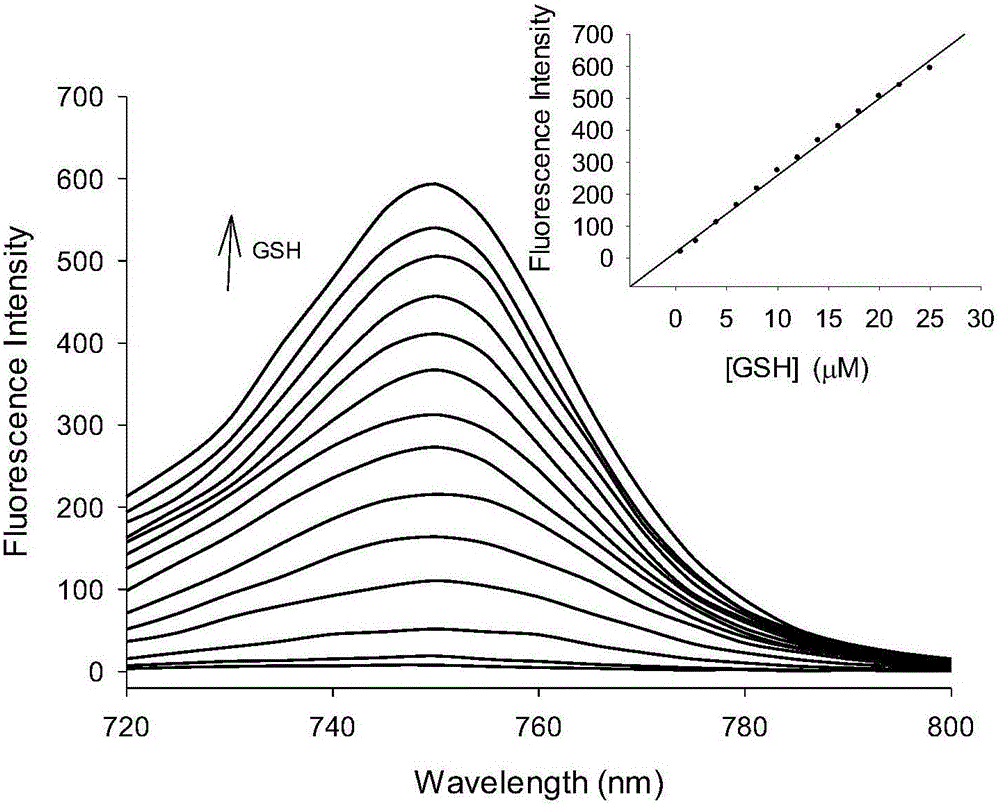
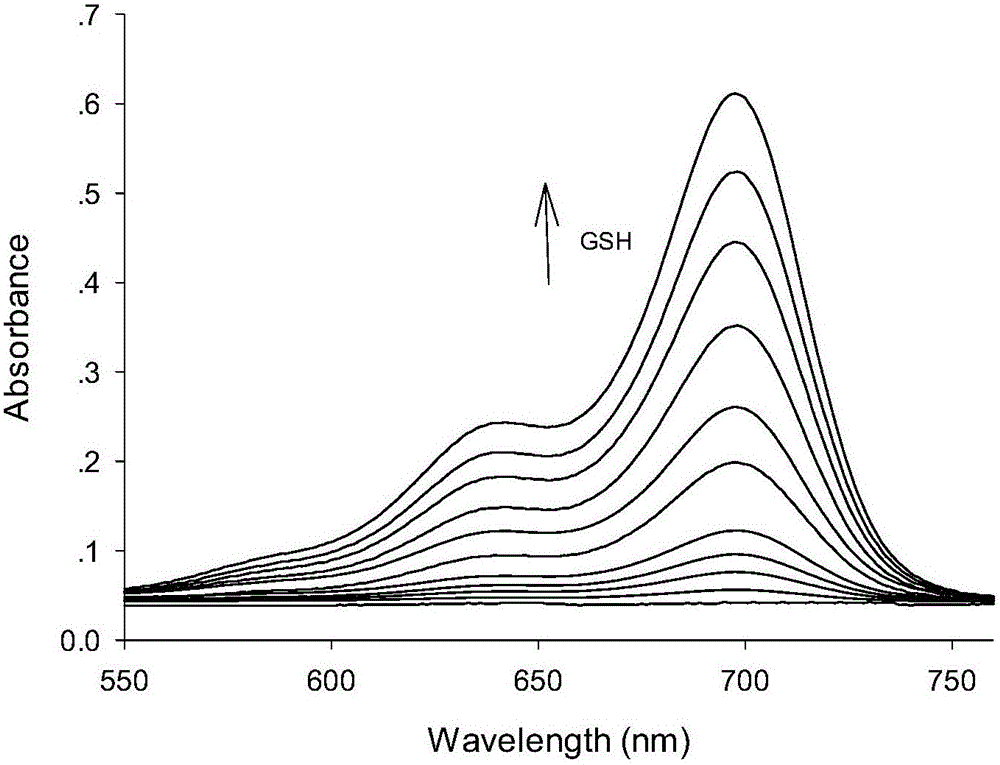
[0042] A buffer solution with a pH value of 7.4 was used as a solvent to measure the fluorescence spectrum of the fluorescent probe interacting with GSH, and the results were as follows: figure 1 . The concentration of the fluorescent probe is 10 μM, the concentration of GSH is 0, 0.5, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0, 16.0, 18.0, 20.0, 22.0, 25.0 μM, the excitation wavelength is fixed at 690 nm, and the emission wavelength The range is 720-800nm, and the slit width is 5.0nm / 5.0nm. From figure 1 It can be seen that before adding GSH, the fluorescent probe has no fluorescence emission peak at 750 nm. With the addition of GSH, the emission peak at 750nm is greatly enhanced, and with the increase of GSH concentration, the fluorescence intensity of the probe is continuously enhanced. When 20 μM GSH is added, the fluorescence intensity is enhanced to 64 times. This is because the ald...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com