A kind of furyl glycidyl ether and its synthesis method and application
A technology of glycidyl ether and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as easy cracks, brittleness, and large internal stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
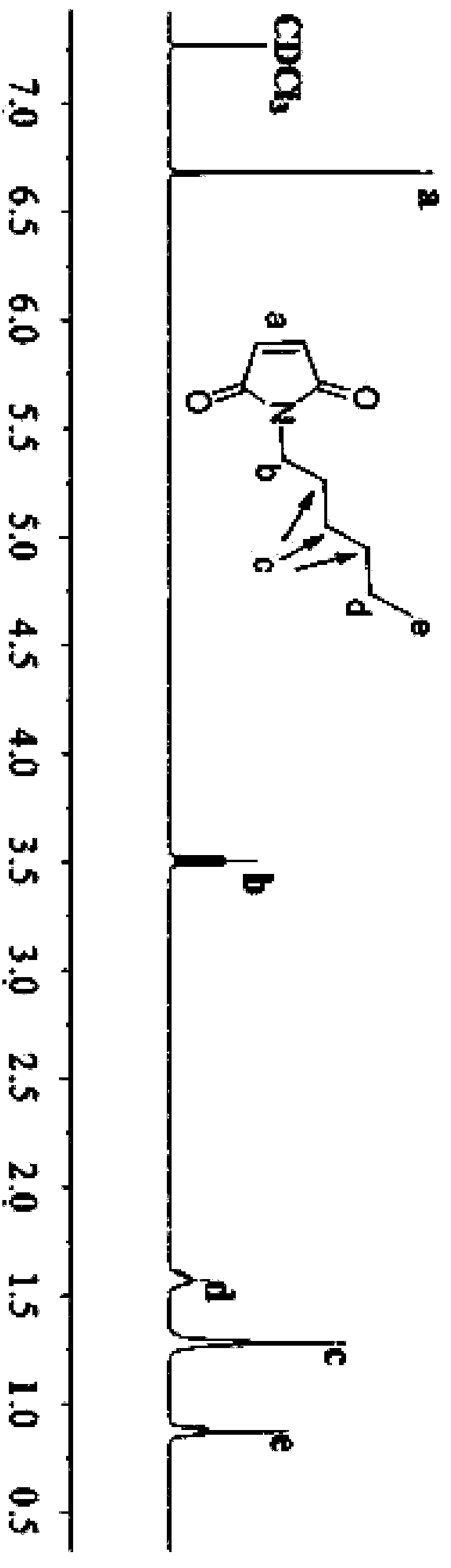
[0049] (1) Mix 100g of maleic anhydride, 1000g of dichloromethane, and 100g of n-hexylamine evenly, react at 30°C for 8 hours, add 150g of oxalyl chloride dropwise to the reaction solution, react at 30°C for 168 hours, and finally in the reaction solution 150g of triethylamine was added dropwise, reacted at 30°C for 24 hours, extracted three times with dichloromethane, washed with saturated brine three times, dried, and dichloromethane was distilled off under reduced pressure to obtain N-hexylmaleimide;
[0050] The proton nuclear magnetic resonance spectrum of the N-hexylmaleimide that prepares is as figure 2 shown;
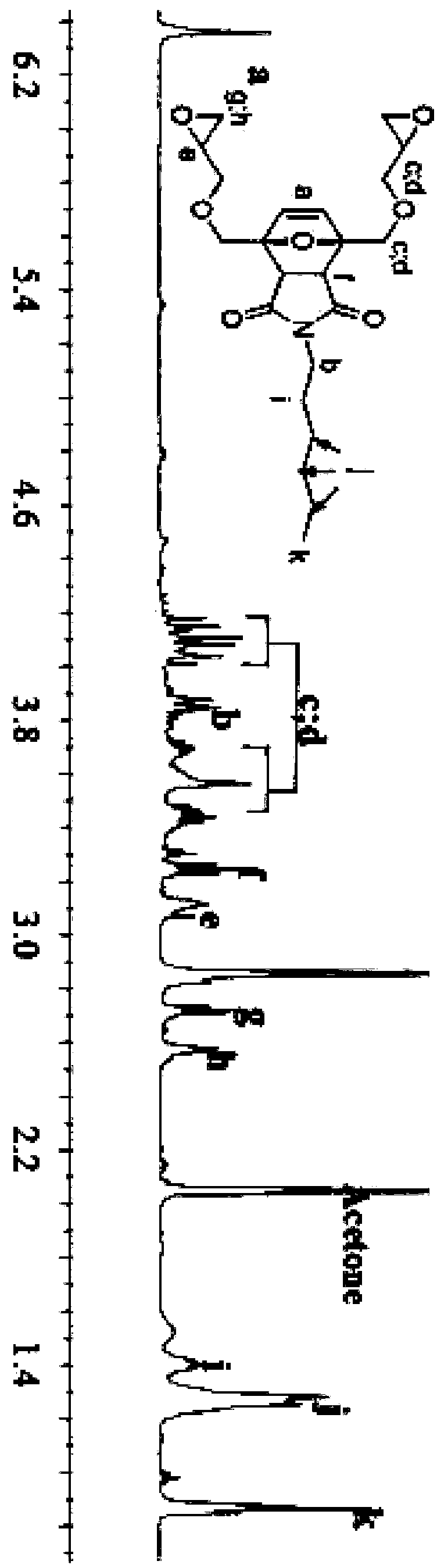
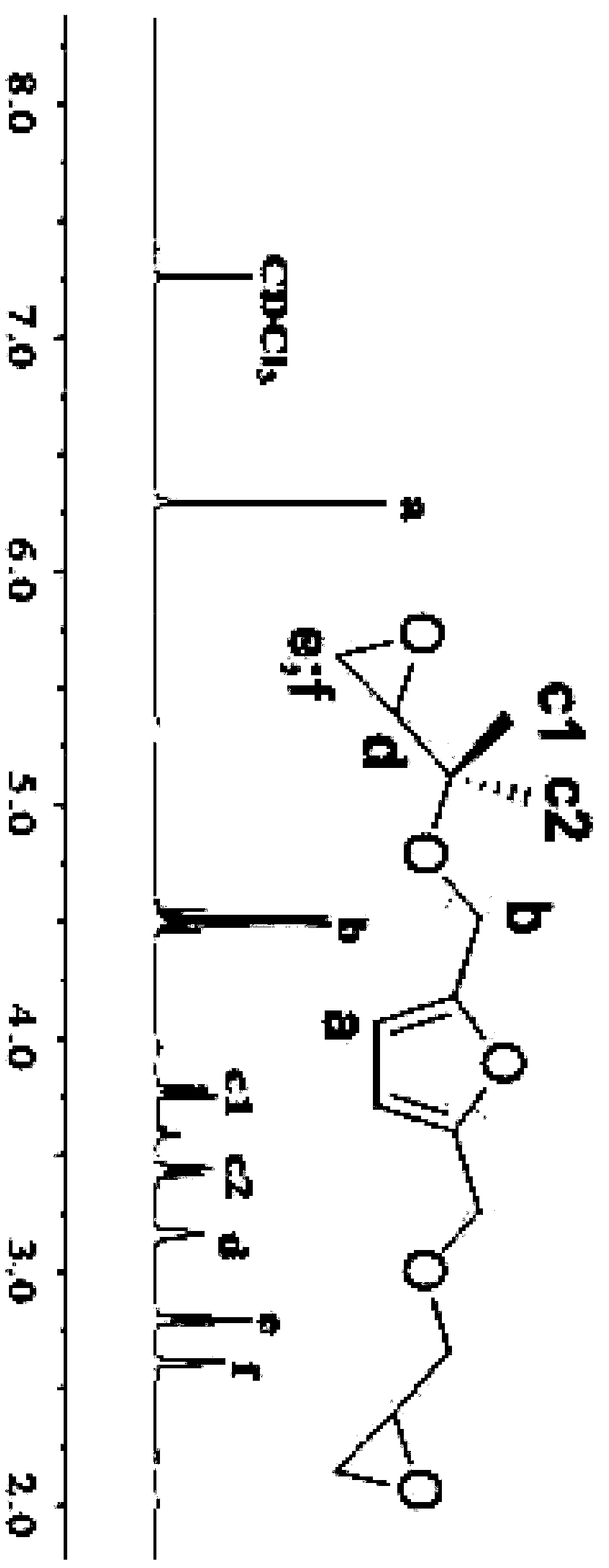
[0051] (2) 100g N-hexylmaleimide, 500g chloroform and 150g furandimethanol glycidyl ether were reacted at 30°C for 24 hours, and the chloroform was removed by distillation under reduced pressure to obtain the formula (I) The furyl glycidyl ether shown, wherein the R group is n-hexyl.
[0052] The yield of the product was 70%, and the epoxy value of the product ...
Embodiment 2
[0055] (1) Mix 100g maleic anhydride, 1000g dichloromethane, and 100g n-hexylamine evenly, react at 40°C for 6 hours, add 200g of oxalyl chloride dropwise to the reaction solution, react at 40°C for 120 hours, and finally in the reaction solution 200g of triethylamine was added dropwise, reacted at 40°C for 24 hours, extracted three times with dichloromethane, washed with saturated brine three times, dried, and dichloromethane was distilled off under reduced pressure to obtain N-hexylmaleimide;
[0056] (2) 100g N-hexylmaleimide, 500g chloroform and 150g furandimethanol glycidyl ether were reacted at 30°C for 24 hours, and the chloroform was removed by distillation under reduced pressure to obtain the formula (I) The furyl glycidyl ether shown, wherein the R group is n-hexyl.
[0057] The yield of the product was 58%, and the epoxy value of the product was 0.46 (theoretical epoxy value was 0.475).
Embodiment 3
[0059] (1) Mix 100g maleic anhydride, 700g acetone, and 150g n-decylamine evenly, react at 40°C for 8 hours, add 100g of acetyl chloride dropwise to the reaction solution, react at 40°C for 240 hours, and finally drop in the reaction solution Add 150g of acetic anhydride, react at 40°C for 50 hours, obtain N-decylmaleimide after extraction, washing with water, drying, and distillation under reduced pressure to remove the solvent;
[0060] (2) 100g N-decylmaleimide, 300g ethyl acetate and 100g furandimethanol glycidyl ether were reacted at 50°C for 24 hours, and the ethyl acetate was distilled off under reduced pressure to obtain formula (I) Shown furyl glycidyl ether, wherein the R group is n-decyl.
[0061] The yield of the product was 55%, and the epoxy value of the product was 0.41 (theoretical epoxy value was 0.419).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com