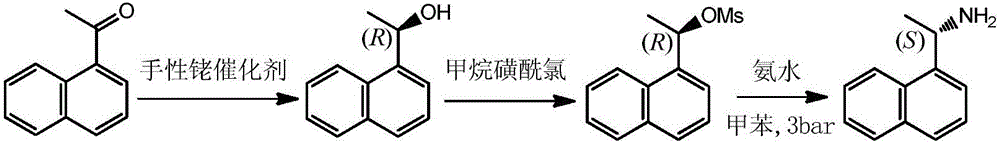
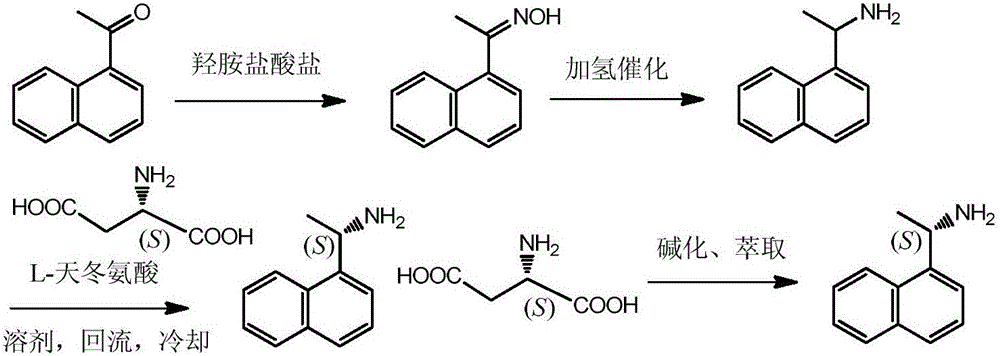
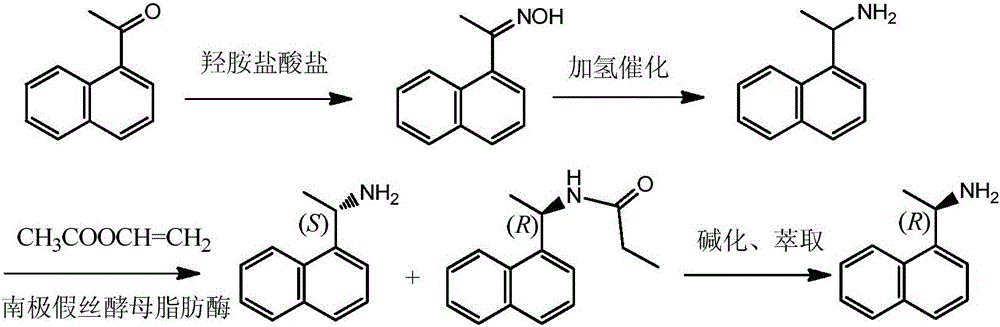
Method for preparing (S)-1-(1-naphthyl)ethylamine by enzymatic resolution
A technology of enzymatic splitting and naphthyl, which is applied in the direction of fermentation, etc., can solve the problems of slow reaction speed of enzymatic method, and achieve the effect of simple process, high splitting efficiency and good high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for enzymatic resolution and preparation of (S)-1-(1-naphthyl)ethylamine has the following steps:
[0026] ① First, prepare 50 mL of Candida antarctica lipase B (hereinafter referred to as CLAB) enzyme solution with a concentration of 1.0 mg / mL, add 150 mg of macroporous resin D3520 to the enzyme solution, stir at 25°C for 3 hours, and remove the resin from the enzyme solution Second, add CLAB-adsorbed resin to 50 mL of 0.5% glutaraldehyde solution, stir and cross-link at 25°C for 2 hours, finally, separate the resin from the glutaraldehyde solution, and wash with pH=7.0 buffer, Immobilized CLAB was obtained after vacuum drying.
[0027] ②In 5mL of toluene solvent, add 5mg of immobilized CLAB prepared in step ①, 2775mg of 1-(1-naphthyl)acetoxime racemate (1.5mmol, hereinafter referred to as racemate) and 264mg of Ethyl acetate (3.0 mmol), 1 g of CuY, and a hydrogen pressure of 0.1 MPa were reacted at a constant temperature of 80° C. for 18 h.
[0028] ③ After...
example 2~ example 5
[0032] The method of each example is basically the same as Example 1, and the differences are shown in Table 1.
[0033] Table 1
[0034]
[0035] As can be seen from Table 1, under other conditions being the same, the immobilized CLAB using adsorbent D151 (Example 3) is the best enzyme catalyst.
Embodiment 6~ Embodiment 17
[0037] Each example is basically the same as Example 3 (that is, the immobilized CLAB of Example 3 is used), and the differences are shown in Table 2.
[0038] Table 2
[0039]
[0040] As can be seen from Table 2, under the situation of adopting the same immobilized enzyme, embodiment 9 (under the temperature of 80 ℃, CuY is racemization catalyst and hydrogen pressure is 0.3MPa) and embodiment 10 resolution effect are the best . But the hydrogen pressure of embodiment 10 is greater than embodiment 9, and following control experiment adopts embodiment 9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com