Nanoparticles preparation encapsulated with carfilzomib, and preparation method thereof
A carfilzomib and nanoparticle technology, which is applied in the field of carfilzomib-encapsulated nanoparticle preparations and the preparation thereof, can solve the problems of poor water solubility, increased toxic and side effects, and is difficult to prepare, and achieves uniform particle size and impurity content. Low and high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0027] The invention provides a nanoparticle preparation loaded with carfilzomib, comprising:
[0028] Carfilzomib 1~20 parts by weight;
[0029] 0.5-10 parts by weight of lecithin;
[0030] 10~100 parts by weight of polyethylene glycol block copolymer;
[0031] The polyethylene glycol block copolymer is a PEG-PLGA-PEG triblock copolymer with a number average molecular weight of 5000-50000 and / or a PEG-PLGA block copolymer with a number average molecular weight of 5000-50000.
[0032] The present invention selects specific polyethylene glycol block copolymers, and uses lecithin as a surfactant, and the water solubility of the prepared carfilzomib nanoparticles is better than that of cyclodextrin inclusion carfilzomib. Higher water solubility and lower impurity content in the preparation.
[0033] In the nanoparticle preparation carrying carfilzomib provided by the present invention, the content of the carfilzomib is preferably 1-20 parts by weight, more preferably 5-15 part...
Embodiment 1
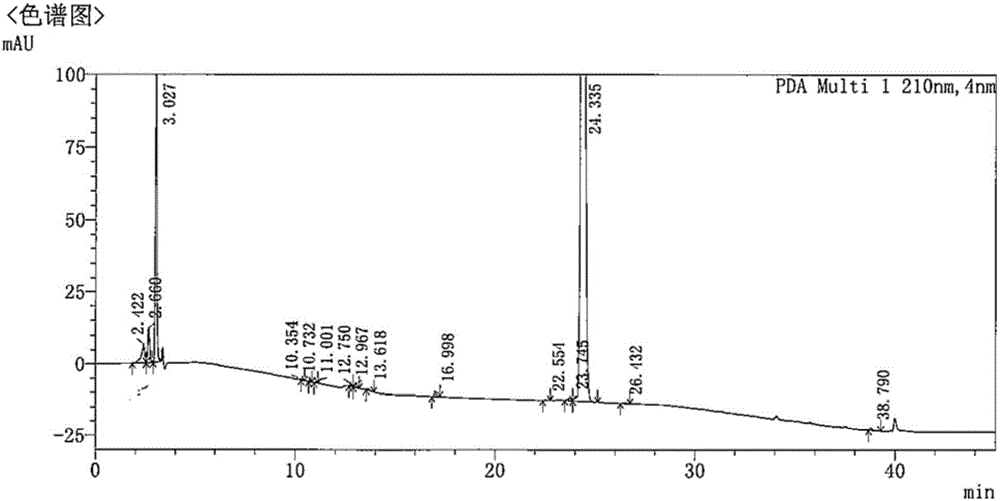
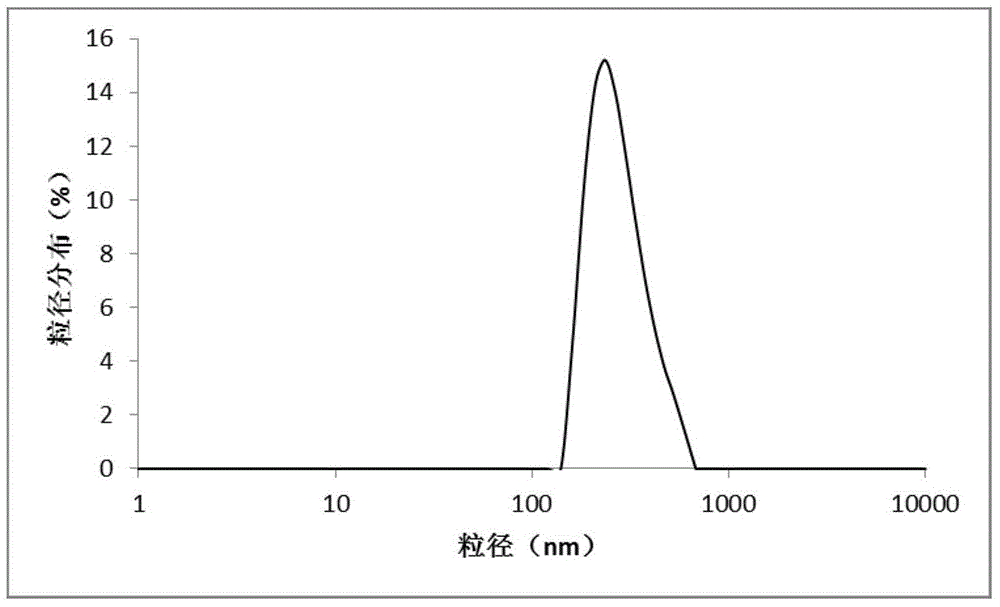
[0061] Dissolve 5 mg of carfilzomib, 2.5 mg of lecithin, 50 mg of mPEG-PLGA in 5 mL of dichloromethane as an organic phase, dissolve poloxamer at a concentration of 2 mg / mL, and dissolve hypromellose at a concentration of 0.4 mg / mL in 50 mL of purified water was added to obtain the aqueous phase. After the organic phase was added to the water phase, it was sheared at 15,000 rpm for 3 minutes with a high-speed emulsification shearer to obtain a carfilzomib nanoemulsion. The emulsion was placed in a fume hood overnight and stirred to remove the organic phase to obtain carfilzomib nanoparticle suspension. After the suspension was removed by centrifugation to remove free drug and surfactant, a nanoparticle preparation loaded with carfilzomib was obtained, which was stored in a refrigerator at 4°C. Gained nanoparticle mean particle size is 265nm, and its particle size distribution figure is shown in attached figure 1 ; The drug loading capacity is 11.08%, and the encapsulation ef...
Embodiment 2
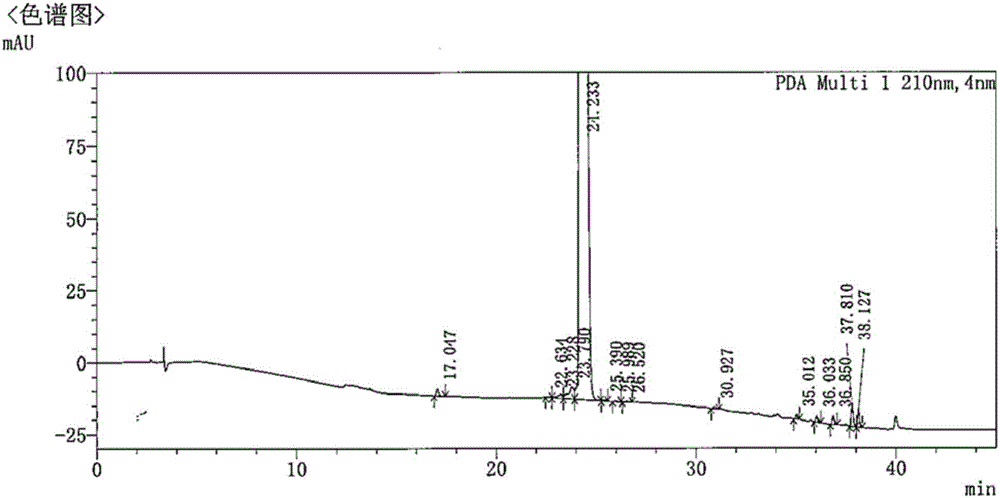
[0073] Dissolve 5 mg of carfilzomib and 50 mg of PEG-PLGA-PEG triblock copolymer in 5 mL of dichloromethane as an organic phase, and dissolve poloxamer in purified water at a concentration of 2 mg / mL to obtain an aqueous phase. After the organic phase was added to the water phase, it was sheared at 15,000 rpm for 3 minutes with a high-speed emulsification shearer to obtain a carfilzomib nanoemulsion. The emulsion was placed in a fume hood overnight and stirred to remove the organic phase to obtain carfilzomib nanoparticle suspension. The suspension was centrifuged to remove free drug and surfactant, and stored in a refrigerator at 4°C. The average particle diameter of the obtained nanoparticles is 180nm, the drug loading capacity is 10.32%, and the encapsulation efficiency is 85.35%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com