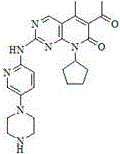
Preparation method for Palbociclib
A technology of pyridine and methyl, which is applied in the field of preparation of the anticancer drug palbociclib, which can solve the problems of difficult synthesis of organic phosphine ligands, increased difficulty of use, harsh reaction conditions, etc., and achieves low price and short production cycle , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
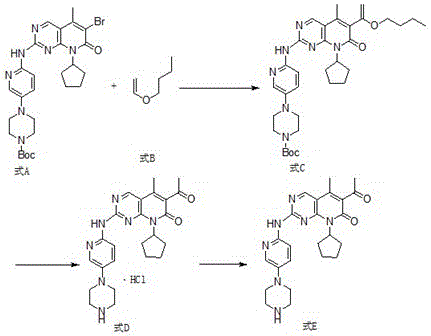
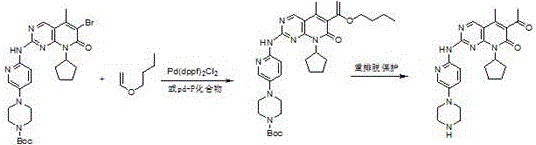
[0046] Step 1: 4-{6-[6-(1-Butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2, Synthesis of 3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylate tert-butyl ester
[0047] 4-[6-(6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-2-ylamino) -pyridin-3-yl]-piperazine-1-carboxylic acid tert-butyl ester (9.8g, 0.017mol), DMF (98ml), vinyl n-butyl ether (17g, 0.17mol), K 2 CO 3 (5.27g, 0.041mol), Pd 2 dba 3 (1.6g, 0.17mmol) and DABCO (0.23 g, 2.04mmol) were added to the reaction flask, heated to 80-85°C for 10h, TLC detected that the reaction was over, cooled to 30°C, filtered, added 98ml of purified water, and crystallized , suction filtration, and vacuum drying at 60°C for 5h to obtain 4-{6-[6-(1-butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8- Dihydro-pyrido[2,3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylic acid tert-butyl ester (9.5 g, yield 94%).
[0048] Step 2: 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-y...
Embodiment 2
[0053] Step 1: 4-{6-[6-(1-Butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2, Synthesis of 3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylate tert-butyl ester
[0054] 4-[6-(6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-2-ylamino) -pyridin-3-yl]-piperazine-1-carboxylic acid tert-butyl ester (5.8g, 0.010mol), DMF (58ml), vinyl n-butyl ether (10g, 0.10mol), K 2 CO 3 (3.3g, 0.024mol), Pd 2 dba 3 (0.091g, 0.10mmol) and DABCO (0.13 g, 112.17, 1.2mmol) were added to the reaction flask, heated to 80-85°C for 12h, TLC detected the end of the reaction, cooled to about 20°C, filtered, and added 58ml of purified water , crystallization, suction filtration, and vacuum drying at 60°C for about 5h to obtain 4-{6-[6-(1-butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo- tert-butyl 7,8-dihydro-pyrido[2,3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylate (5.6g, yield 93%) .
[0055] Step 2: 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(pi...
Embodiment 3
[0060] Step 1: 4-{6-[6-(1-Butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2, Synthesis of 3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylate tert-butyl ester
[0061] 4-[6-(6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-2-ylamino) -pyridin-3-yl]-piperazine-1-carboxylic acid tert-butyl ester (9.8g, 0.017mol), DMF (98ml), vinyl n-butyl ether (17g, 0.17mol), K 2 CO 3 (5.27g, 0.041mol), Pd 2 dba 3 (1.6g, 0.17mmol) and DABCO (0.23 g, 2.04mmol) were added to the reaction flask, heated to 80-85°C for 10h, TLC detected the end of the reaction, cooled to 15°C, filtered, added 58ml of purified water, and crystallized , suction filtration, and vacuum drying at 60°C for 5h to obtain 4-{6-[6-(1-butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8- Dihydro-pyrido[2,3-d]pyrimidin-2-ylamino)-pyridin-3-yl}-piperazine-1-carboxylic acid tert-butyl ester (9.5 g, yield 94%).
[0062] Step 2: 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com