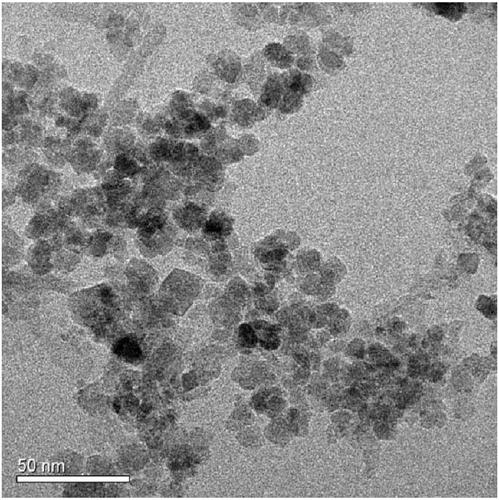
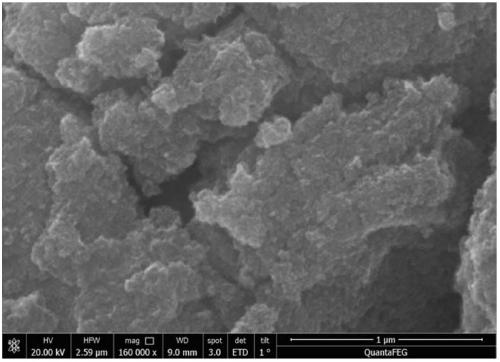
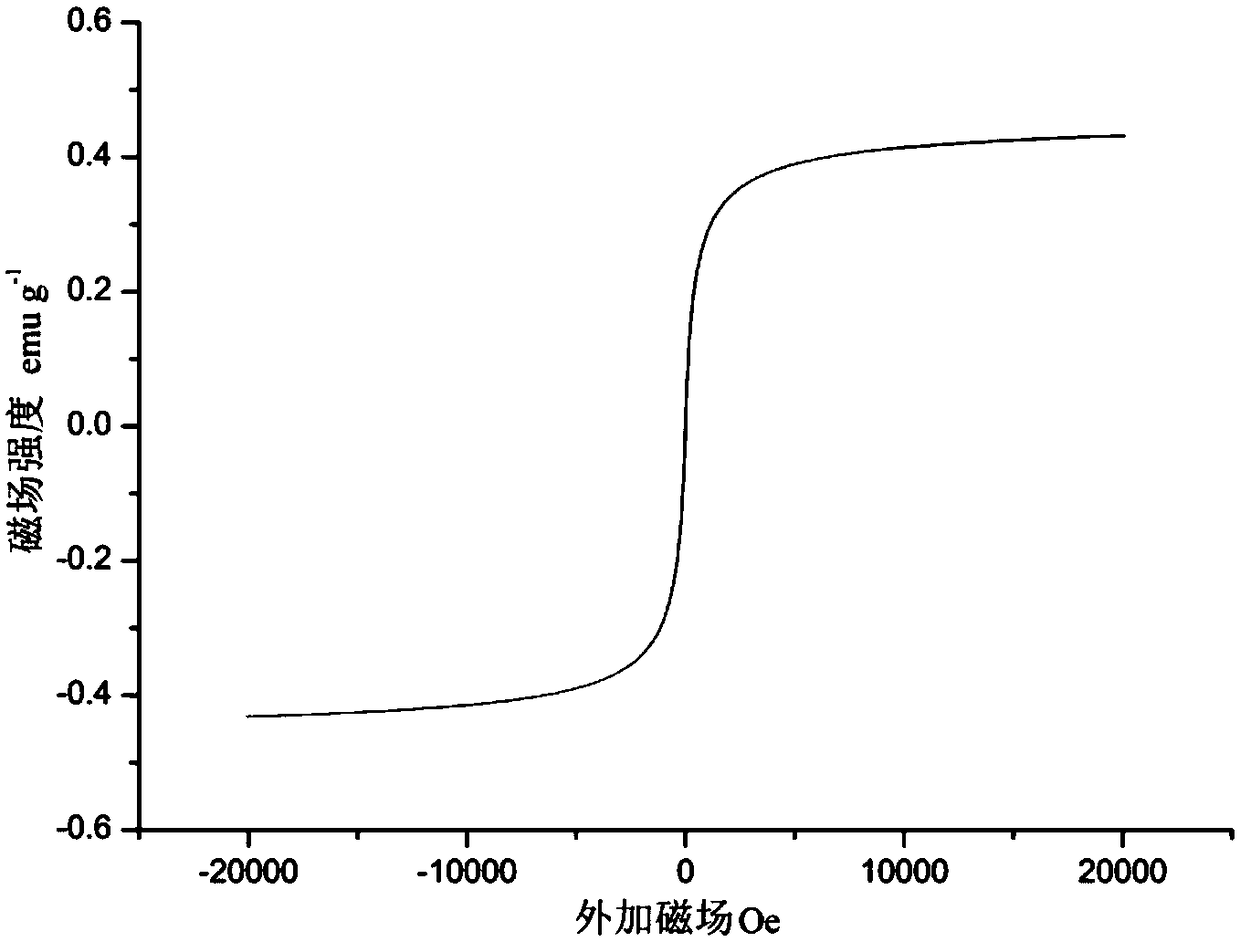
Method for removing heavy metals in wastewater by using magnetic nano-chloroapatite adsorbent
A technology of chloroapatite and magnetic nano, which is applied in the direction of adsorption of water/sewage treatment, alkali metal compounds, chemical instruments and methods, etc., and can solve problems such as application limitations of nanomaterials, difficulty in solid-liquid separation, and eutrophication of water bodies. Achieve the effect of remarkable removal effect, wide application prospect and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of the magnetic nanometer chloroapatite adsorbent that the inventive method adopts comprises the following steps:
[0037] (1) 1.85mmol of FeCl 2 4H 2 O and 3.7 mmol FeCl 3 ·6H 2 O was dissolved in 30 mL of deoxygenated water and sonicated for 10 min. Under the condition of continuous stirring, 10 mL of ammonia solution with a concentration of 25% was added to obtain a mixed solution.
[0038] (2)CaCl 2 Solution preparation: weigh 1.9698g CaCl 2 2H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volumetric flask, use ultrapure water to make up the volume to the mark, and shake well to obtain a concentration of 26.8mM CaCl 2 solution.
[0039] Na 3 PO 4 Solution preparation: weigh 3.04g Na 3 PO 4 12H 2 0 into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volumetric flask, use ultrapure water to make the volume up to the mark, and shake well t...
Embodiment 2
[0046] A kind of method utilizing magnetic nano chloroapatite adsorbent of the present invention to remove heavy metals in waste water comprises the following steps:
[0047] (a) Take three kinds of heavy metal ions (Pb 2+ 、Cd 2+ , Zn 2+ ) concentration of 2mM wastewater, and adjust the pH value of the wastewater to 10.0±0.1 with 1M NaOH solution.
[0048] (b) Add the magnetic nano-chloroapatite adsorbent in Example 1 to the waste water of 20mL step (a) respectively, the consumption of adsorbent is 1g / L, shake at a constant temperature of 25°C, and the oscillation speed is 120r / min . After 24 hours, the magnetic nano-chloroapatite adsorbent was separated from the above solution with a magnet to remove heavy metal ions in the wastewater; Unadsorbed Pb 2+ 、Cd 2+ , Zn 2+ content. The measurement results are shown in Table 1.
[0049] Table 1: Removal rate of heavy metals in wastewater
[0050] Heavy Metal Ions in Wastewater
Embodiment 3
[0052] A kind of method utilizing magnetic nano chloroapatite adsorbent of the present invention to remove heavy metals in waste water comprises the following steps:
[0053] (a) Take three kinds of heavy metal ions (Pb 2+ 、Cd 2+ , Zn 2+ ) concentration of 2mM wastewater, and adjust the pH value of the wastewater to 5.0±0.1 with 1M HCl solution respectively.
[0054] (b) Add the magnetic nano-chloroapatite adsorbent of Example 1 to 20mL of the waste water of step (a) respectively, the amount of adsorbent is 1g / L, shake at a constant temperature of 25°C, and the oscillation speed is 120r / min. After 24 hours, the magnetic nano-chloroapatite adsorbent was separated from the above solution with a magnet to remove heavy metal ions in the wastewater; Unadsorbed Pb 2+ 、Cd 2+ , Zn 2+ content. The measurement results are shown in Table 2.
[0055] Table 2: Removal rate of heavy metals in wastewater
[0056] Heavy Metal Ions in Wastewater
[0057] From the experimenta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com