Imidazolium bromide ionic liquid containing amine groups and preparation method and application of ionic liquid
An imidazolium bromide, ionic liquid technology, applied in chemical instruments and methods, catalytic reactions, organic chemistry, etc., to reduce costs, ensure catalytic effects, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
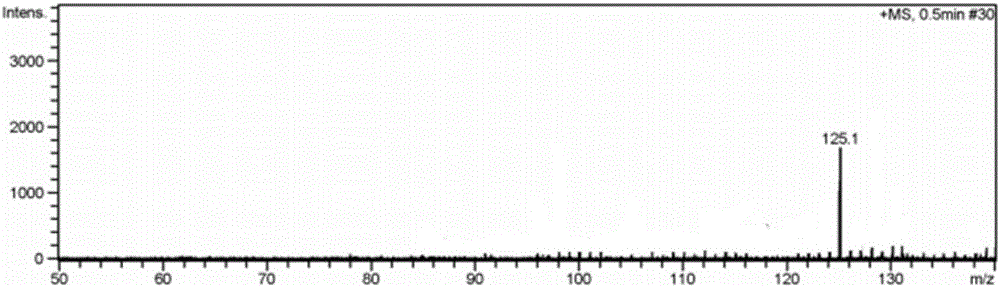
[0022] Embodiment 1 has the preparation of the imidazolium bromide salt ionic liquid of monoamino
[0023] The structural formula of the imidazolium bromide salt ionic liquid with monoamino is as follows:
[0024]
[0025] R=CH 3 , C 4 h 9 , CH 2 = CH; n = 2 or 3
[0026] (1) 1-aminopropyl-3-butyl imidazolium bromide ([NH 2 Preparation of pbim]Br)
[0027] Under the protection of nitrogen, mix 3-bromopropylamine hydrobromide and n-butylimidazole at a molar ratio of 1:1.1, dissolve in a certain amount of absolute ethanol solution, reflux at 90°C for 24 hours, and distill under reduced pressure After the ethanol solvent, add dichloromethane solvent, stir, filter, distill the filtrate under reduced pressure to remove dichloromethane, add an appropriate amount of KOH aqueous solution after vacuum drying to make the solution pH 8-9, filter, add absolute ethanol solvent to the solid, Stir and dissolve, filter, and after the filtrate is vacuum-dried, pure 1-aminopropyl-3-bu...
Embodiment 2
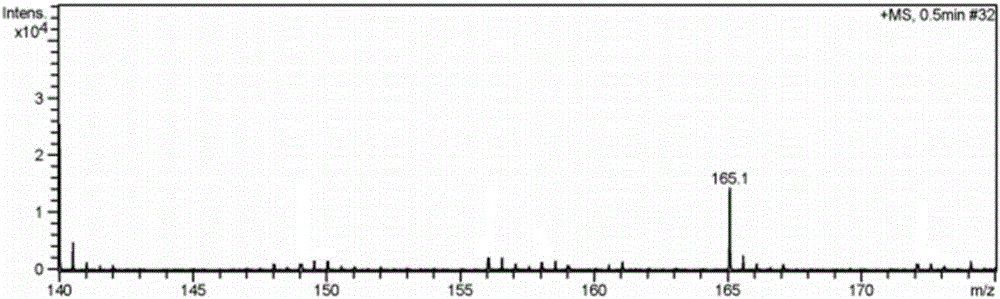
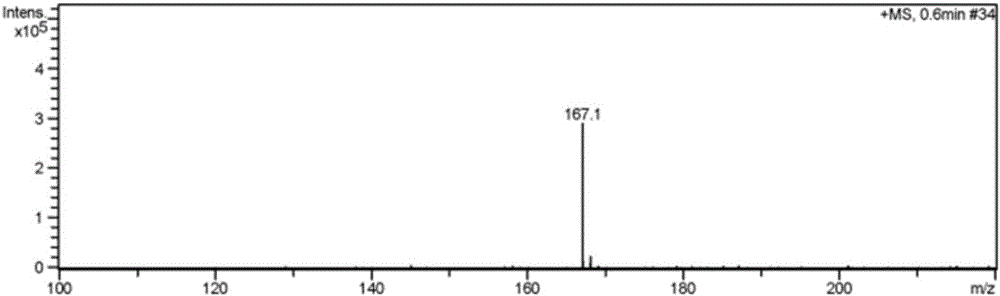
[0034] Embodiment 2 has the preparation of the imidazolium bromide salt ionic liquid of two amino groups
[0035] The structural formula of the imidazolium bromide salt ionic liquid with two amino groups is as follows:
[0036]
[0037] R=(CH 2 ) n NH 2 ;n=2 or 3
[0038] (1) 1,3-diaminopropylimidazolium bromide ([2NH 2 pim]Br) preparation:
[0039] Under the protection of nitrogen, mix 3-bromopropylamine hydrobromide and trimethylsilyl imidazole at a molar ratio of 1:2.2, dissolve in a certain amount of absolute ethanol solution, condense and reflux at 90°C for 24 hours, and the reaction solution From clarification to light yellow transparent viscous liquid gradually, after vacuum distillation to remove ethanol solvent, add toluene solvent, stir, filter, distill the filtrate to remove toluene under reduced pressure, add appropriate amount of KOH aqueous solution after vacuum drying to make the solution pH 8-9, Filtrate, add dehydrated ethanol solvent in the solid, st...
Embodiment 3
[0042] Embodiment 3 has the imidazolium bromide salt ionic liquid of amino group to catalyze epoxy compound cycloaddition reaction
[0043] (1) The influence of temperature on the reaction yield
[0044] In a 50ml autoclave, add the catalyst [NH 2 pbim]Br and epichlorohydrin, mixed, passed into 5MP CO 2 , as shown in Table 1, the temperature was reacted for 2 hours. The addition amount of catalyst is 0.6% of the mole number of epichlorohydrin. Calculate the productive rate of cyclic carbonate after the reaction finishes, and the results are shown in Table 1.
[0045] Table 1
[0046] temperature °C
60
80
100
120
140
Yield%
39.6
70.7
82.4
87.5
99.9
[0047] (2) CO 2 Effect of Pressure on Reaction Yield
[0048] The method is the same as (1), the temperature is 140°C, the pressure is changed as shown in Table 2, and the results are shown in Table 2.
[0049] Table 2
[0050] Pressure MP
1
3
5
Yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com