Mezlocillin sodium and sulbactam sodium compound drug composition for injection
A technology for mezlocillin sodium sulbactam sodium and cillin sodium sulbactam sodium, applied in the field of medicine, can solve the problems of high incidence of adverse reactions, large polymer, unsafe medication and the like, and achieves low incidence of adverse reactions, The effect of low polymer content and uniform mixing of main drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: mezlocillin sodium compound pharmaceutical composition of the present invention
[0033] 1. Components
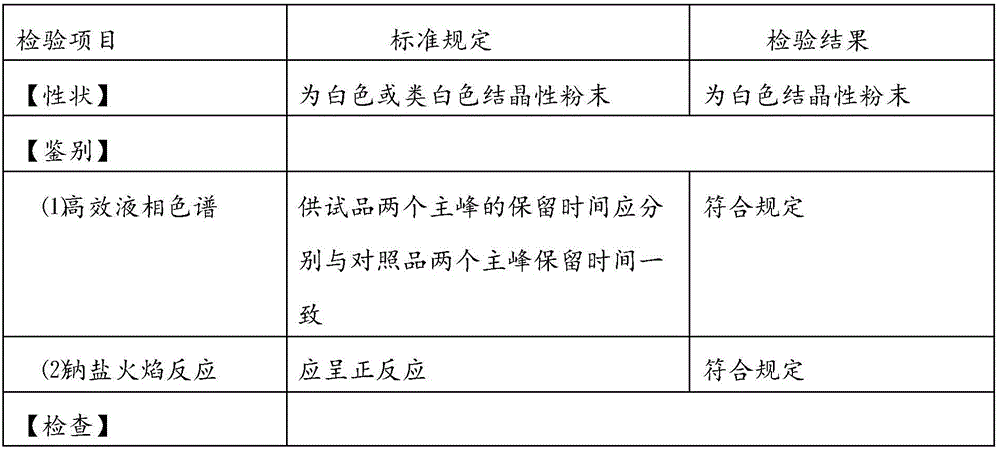
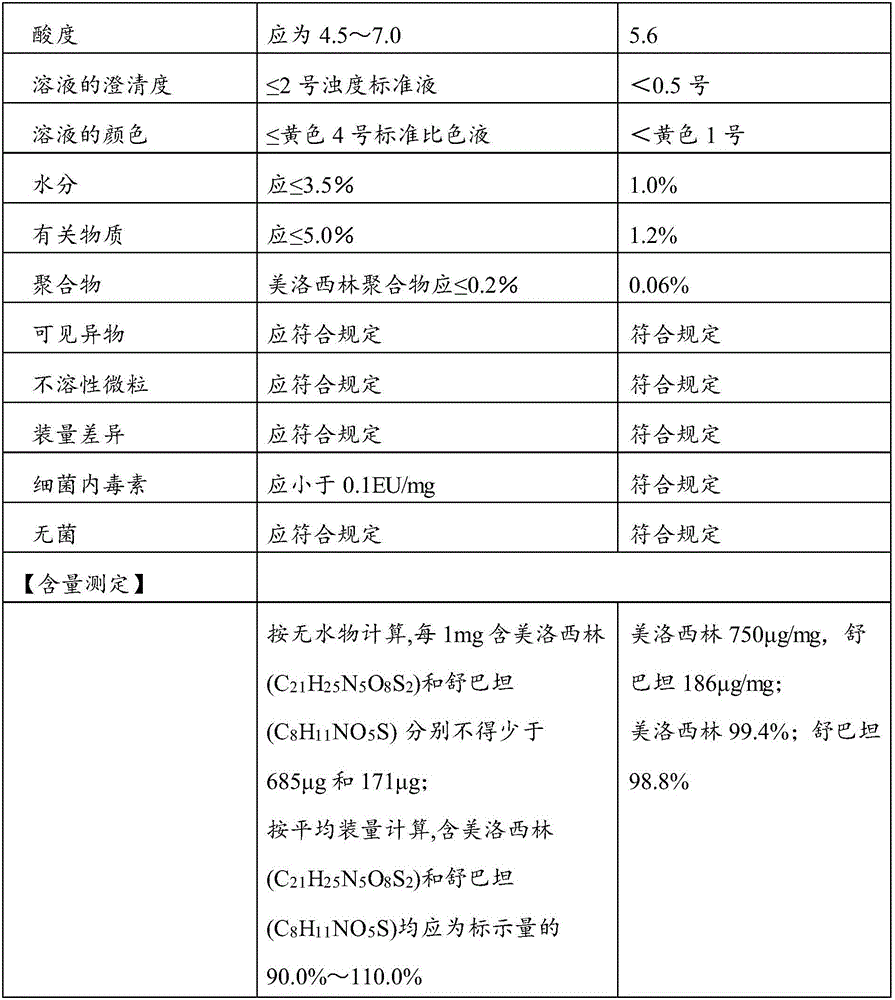
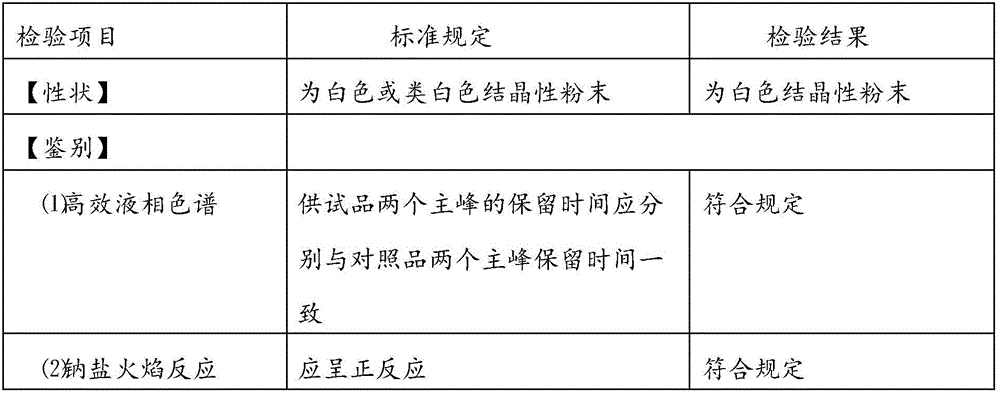
[0034] 10 parts of mezlocillin sodium (calculated as mezlocillin), 2.5 parts of sulbactam sodium (calculated as sulbactam), 0.20 parts of sodium benzoate.
[0035] 2. Preparation method
[0036] Add 20Kg of water for injection at 6-10°C to the reaction kettle, add 10Kg of mezlocillin, turn on the stirring device and cooling device, add dropwise sodium bicarbonate suspension (1.60Kg of sodium bicarbonate is added to 6-10°C and 15Kg of water for injection Middle), keep the reaction temperature at 6°C, take the clarified medicinal solution to check the pH value, the pH value should be 6.0-7.3, and after the pH value of the medicinal solution is stable (the deviation of the pH value of the medicinal solution for three consecutive times is within ±0.03), add Dissolved sulbactam sodium solution [2.5Kg sulbactam sodium (calculated as sulbactam) dissolved in ...
Embodiment 2
[0044] Embodiment 2: mezlocillin sodium compound pharmaceutical composition of the present invention
[0045] 1. Components
[0046] 10 parts of mezlocillin sodium (calculated as mezlocillin), 2.5 parts of sulbactam sodium (calculated as sulbactam), 0.25 parts of sodium benzoate.
[0047] 2. Preparation method
[0048] Add 20Kg of water for injection at 6-10°C to the reaction kettle, add 10Kg of mezlocillin, turn on the stirring device and cooling device, add dropwise sodium bicarbonate suspension (1.80Kg of sodium bicarbonate is added to 6-10°C and 15Kg of water for injection Middle), keep the reaction temperature at 8°C, take the clarified medicinal solution to check the pH value, the pH value should be 6.0-7.3, and after the pH value of the medicinal solution is stable (the deviation of the pH value of the medicinal solution for three consecutive times is within ±0.03), add Dissolved sulbactam sodium solution [2.5Kg sulbactam sodium (calculated as sulbactam) dissolved in ...
Embodiment 3
[0056] Embodiment 3: mezlocillin sodium compound pharmaceutical composition of the present invention
[0057] 1. Components
[0058] 10 parts of mezlocillin sodium (calculated as mezlocillin), 2.5 parts of sulbactam sodium (calculated as sulbactam), 0.30 parts of sodium benzoate.
[0059] 2. Preparation method
[0060] Add 20Kg of water for injection at 6-10°C to the reaction kettle, add 10Kg of mezlocillin, turn on the stirring device and cooling device, add dropwise sodium bicarbonate suspension (2.0Kg of sodium bicarbonate is added to 6-10°C and 15Kg of water for injection Middle), keep the reaction temperature at 10°C, take the clarified medicinal solution to check the pH value, the pH value should be 6.0-7.3, and after the pH value of the medicinal solution is stable (the deviation of the pH value of the medicinal solution for three consecutive times is within ±0.03), add Dissolved sulbactam sodium solution [2.5Kg sulbactam sodium (calculated as sulbactam) dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com