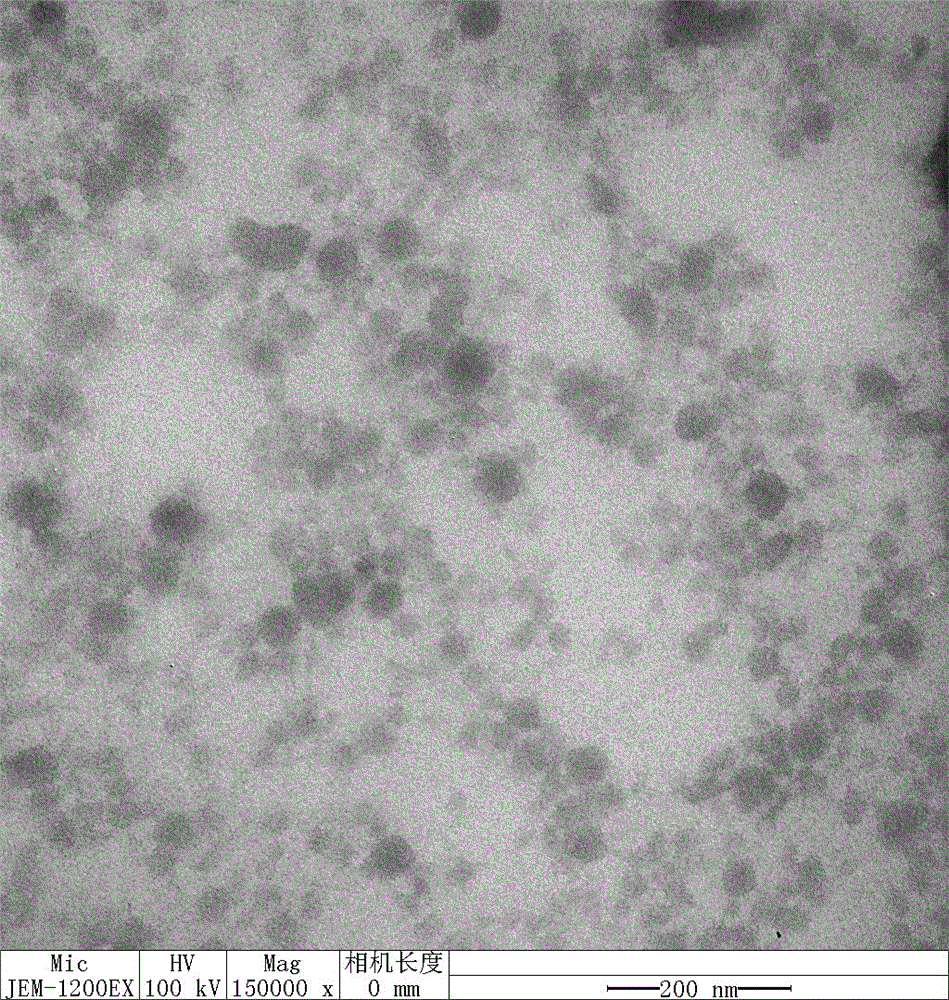
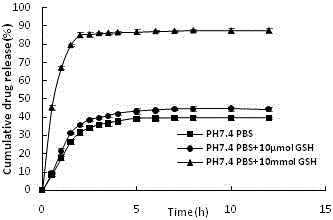
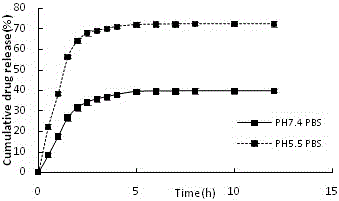
Preparation method of amycin controlled-release chitosan nano particles with pH/oxido-reduction dual response
A technology of chitosan nanoparticles and doxorubicin, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of drug structure destruction, escape, inactivation, etc. It achieves the effects of convenient operation, improved conveying efficiency, and simple preparation technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh 2 mg of mercapto-chitosan, dissolve it in 2 mL of 1 mM hydrochloric acid solution, add dropwise 0.3 mL of 1 mg / ml doxorubicin hydrochloride solution under stirring conditions, and obtain mixed solution 1, and take 3 mg of it with a molecular weight of 150 kDa Carboxymethyl chitosan was dissolved in 3 mL of deionized water, and 0.1 mL of sodium tripolyphosphate solution with a concentration of 2% (w / v) was added dropwise under stirring conditions to obtain mixed solution 2, and mixed solution 1 was added dropwise Into the mixed solution 2, the dropping rate is 30 drops / min, adjust the pH value to pH 7.5 with hydrochloric acid, stir and react at room temperature for 1 h, ultrasonically mix, ultrasonic power 200 W, time 5 min, open for 2 s, stop for 1 s, Add 0.05 mL of 30% hydrogen peroxide dropwise under stirring conditions, stir the reaction at room temperature for 2 h, centrifuge at 15,000 rpm for 30 min, remove the supernatant, and freeze-dry to obtain the doxorub...
Embodiment 2
[0019] Weigh 2 mg of mercaptochitosan, dissolve it in 2 mL of 1 mM hydrochloric acid solution, add dropwise 0.3 mL of 1 mg / ml doxorubicin hydrochloride solution under stirring conditions, and obtain mixed solution 1, take 4 mg of the molecular weight of 400 kDa Carboxymethyl chitosan was dissolved in 4 mL of deionized water, and 0.2 mL of sodium tripolyphosphate solution with a concentration of 2% (w / v) was added dropwise under stirring conditions to obtain mixed solution 2, and mixed solution 1 was added dropwise Into the mixed solution 2, the dropping speed is 30 drops / min, adjust the pH value to pH 8.0 with hydrochloric acid, stir and react at room temperature for 1 h, mix with ultrasonic, ultrasonic power 200 W, time 5 min, open for 2 s, stop for 1 s, Add 0.05 mL of 30% hydrogen peroxide dropwise under stirring conditions, stir the reaction at room temperature for 2 h, centrifuge at 15,000 rpm for 30 min, remove the supernatant, and freeze-dry to obtain the doxorubicin cont...
Embodiment 3
[0021] Weigh 2 mg of mercapto-chitosan, dissolve it in 2 mL of 1 mM hydrochloric acid solution, add dropwise 0.3 mL of 1 mg / ml doxorubicin hydrochloride solution under stirring conditions, and obtain mixed solution 1, take 3 mg of the molecular weight of 1500 kDa Dissolve carboxymethyl chitosan in 3 mL deionized water, add 0.5 mL sodium tripolyphosphate solution with a concentration of 2% (w / v) dropwise under stirring conditions to obtain mixed solution 2, add mixed solution 1 dropwise Into the mixed solution 2, the dropping rate is 30 drops / min, adjust the pH value to pH 6.0 with hydrochloric acid, stir and react at room temperature for 1 h, mix with ultrasonic, ultrasonic power 200 W, time 5 min, open for 2 s, stop for 1 s, Add 0.05 mL of 30% hydrogen peroxide dropwise under stirring conditions, stir the reaction at room temperature for 2 h, centrifuge at 15,000 rpm for 30 min, remove the supernatant, and freeze-dry to obtain the doxorubicin control with pH / redox double respo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com