Chitosan-based self-healing gel and preparation method thereof
A chitosan and self-healing technology, which is applied in the field of chitosan-based self-healing gel and its preparation, can solve the problems of less research on natural polymer self-healing gel, and achieve good self-healing properties , mild reaction and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
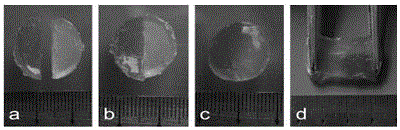
[0024] A certain amount of chitosan was dispersed in a mixed solution of 50 wt% NaOH and 12 wt% urea at -30 o Ice was formed at ℃, placed for 12 hours, and then thawed into a solution at room temperature with a concentration of 0.5 wt%. Adding acrylamide to the chitosan solution at a molar ratio of chitosan unit and acrylamide was 1:1, and the 0 o C for 1 h to obtain acrylamide chitosan with a degree of substitution of 0.12. Dissolve acrylamide chitosan with a substitution degree of 0.12 in ultrapure water to prepare a solution with a concentration of 0.5 wt%, and add an oxidation degree of 5 % oxidized natural polysaccharides, mixed well at room temperature, and after the gel was prepared, it was stored in a desiccator containing saturated sodium chloride to maintain a humidity of 75%. Cut the prepared gel and stick it together. After a certain period of time, take pictures with a camera and observe the healing condition of the gel with an optical microscope.
Embodiment 2
[0026] A certain amount of chitosan was dispersed in a mixed solution of 15 wt% KOH, 8 wt% NaOH, and 12 wt% thiourea at -30 o It was frozen under ℃, placed for 12 hours, then thawed into a solution at room temperature, the concentration was 8 wt%, and the molar ratio of chitosan unit and acrylamide was 1:25 to add acrylamide to the chitosan solution. 15 o C for 72 h to obtain acrylamide chitosan with a degree of substitution of 0.96. Dissolve acrylamide chitosan with a substitution degree of 0.96 in ultrapure water to prepare a solution with a concentration of 0.5 wt%, and add acrylamide chitosan with an oxidation degree of 5 % oxidized natural polysaccharides, mixed well at room temperature, after gel preparation, stored in a desiccator containing saturated sodium chloride to maintain a humidity of 75%. Cut the prepared gel and stick it together. After a certain period of time, take pictures with a camera and observe the healing condition of the gel with an optical microsco...
Embodiment 3
[0028] A certain amount of chitosan was dispersed in a mixed solution of 20 wt% LiOH, 8 wt% NaOH, and 12 wt% urea at -30 o Ice was formed under the temperature of C, placed for 12 hours, and then thawed into a solution at room temperature, the concentration was 3 wt%, and the molar ratio of chitosan unit and acrylamide was 1:25 to add acrylamide to the chitosan solution. 15 o C for 72 h to obtain acrylamide chitosan with a degree of substitution of 0.96. Dissolve acrylamide chitosan with a substitution degree of 0.96 in ultrapure water to prepare a solution with a concentration of 3 wt%, and add acrylamide chitosan with an oxidation degree of 5 % oxidized natural polysaccharides, mixed well at room temperature, after gel preparation, stored in a desiccator containing saturated sodium chloride to maintain a humidity of 75%. Cut the prepared gel and stick it together. After a certain period of time, take pictures with a camera and observe the healing condition of the gel with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com