Azo-benzoic acid type compound as well as preparation method and applications thereof
A technology of azobenzoic acid and benzoic acid, applied in organic chemistry, drug combination, cardiovascular system diseases, etc., can solve undiscovered problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention also provides a method for preparing the compound of the structure represented by formula (1), wherein the method includes:
[0030] (1) In the presence of inorganic acid, the formula (2) The compound of the structure shown and the diazotizing agent undergo a diazotization reaction;
[0031] (2) In the presence of an alkali metal hydroxide, contacting the product mixture obtained from the diazotization reaction with salicylic acid;
[0032] (3) Acidifying the product mixture after the contact reaction;
[0033]
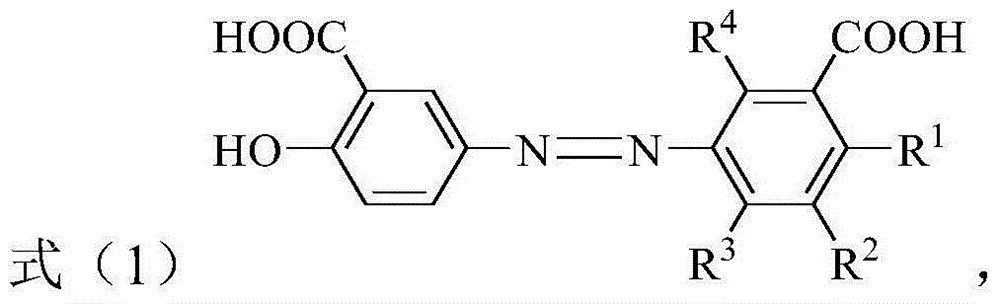
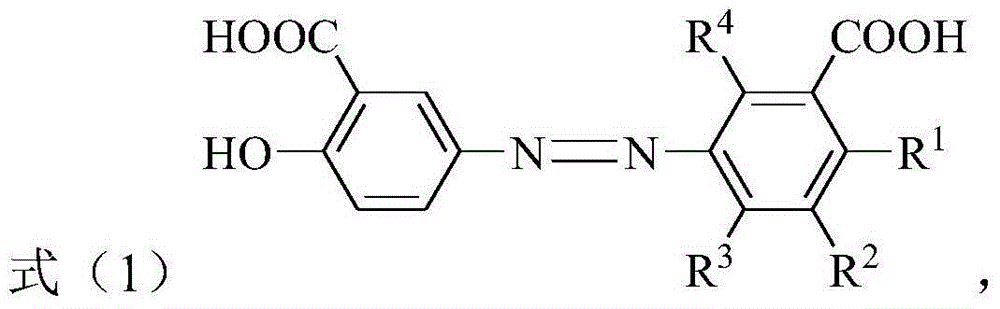
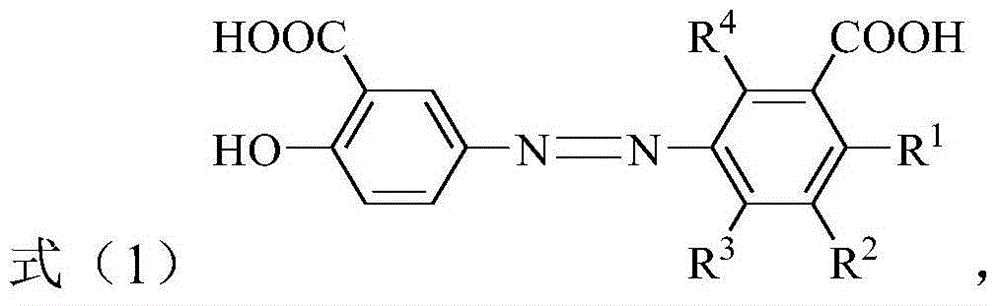
[0034] Where R 1 Is hydrogen, C 1 -C 4 The alkyl group, C 1 -C 4 The alkoxy, halogen, nitro or carboxyl group; R 2 , R 3 And R 4 Each independently is hydrogen, C 1 -C 4 The alkyl group, C 1 -C 4 的alkoxy, C 1 -C 4 The hydroxyalkyl, halogen, nitro or carboxyl group; and R 1 , R 2 , R 3 And R 4 It is not hydrogen at the same time.
[0035] According to the present invention, in the above preparation method, R 1 , R 2 , R 3 And R 4 The preferred combi...
Embodiment 1
[0059] This example is used to illustrate the azobenzoic acid compound of the present invention and the preparation method thereof.
[0060] Stir and mix 5-amino-2-methylbenzoic acid (1.51g, 10mmol) and 6mL of aqueous hydrochloric acid (6mol / L). Add 3.8g of NaNO dropwise while stirring at 0°C 2 Aqueous solution (20% by weight), control the dropping rate so that the temperature does not exceed 10°C, react for 30min to obtain the diazonium salt solution after the addition is complete; continue to add the diazonium salt solution dropwise to the salicylic acid (1.24 g, 9mmol) 10% by weight NaOH aqueous solution (16mL), control the dropping rate so that the temperature does not exceed 10℃, and continue to react at 0℃ for 2h, then add 6mol / L hydrochloric acid aqueous solution to the reaction system until the reaction The pH of the system was 1, filtered, and the filter cake was washed with water to obtain the compound of the formula (1-1) (1.21 g, yield 90%) as a yellow solid. Mp: 295°...
Embodiment 2
[0062] This example is used to illustrate the azobenzoic acid compound of the present invention and the preparation method thereof.
[0063] Stir and mix 4-amino-phthalic acid (0.97g, 5mmol) and 3mL of hydrochloric acid aqueous solution (6mol / L), add 2g of NaNO dropwise while stirring at 0℃ 2 Aqueous solution (20% by weight), control the dropping rate so that the temperature does not exceed 10°C, react for 40 minutes after the addition to obtain the diazonium salt solution; continue to add the diazonium salt solution to the salicylic acid (0.69 g, 10mmol) 10 wt% NaOH aqueous solution (15mL), control the dropping rate so that the temperature does not exceed 10 ℃, and continue to react at 0 ℃ for 3.5h, and then add 5mol / L hydrochloric acid aqueous solution to the reaction system to The pH of the reaction system was 2, filtered, and the filter cake was washed with water to obtain the compound of the formula (1-2) (1.14 g, yield 69%) as a yellow solid. Mp: 298-299°C (decomposition). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com