Optically pure thioacetic compound
A pure compound, thioacetic acid technology, applied in the field of pharmacy, can solve the problem of no chiral enantiomer reporting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Racemic Lesinurad (2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid) The synthesis can refer to the synthesis of documents WO2009070740A2, CN103524440A and WO2014008295A1, and the synthesis route is as follows:
[0047]
Embodiment 2
[0048] Example 2: Optically pure single enantiomer 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio) Preparation of acetic acid
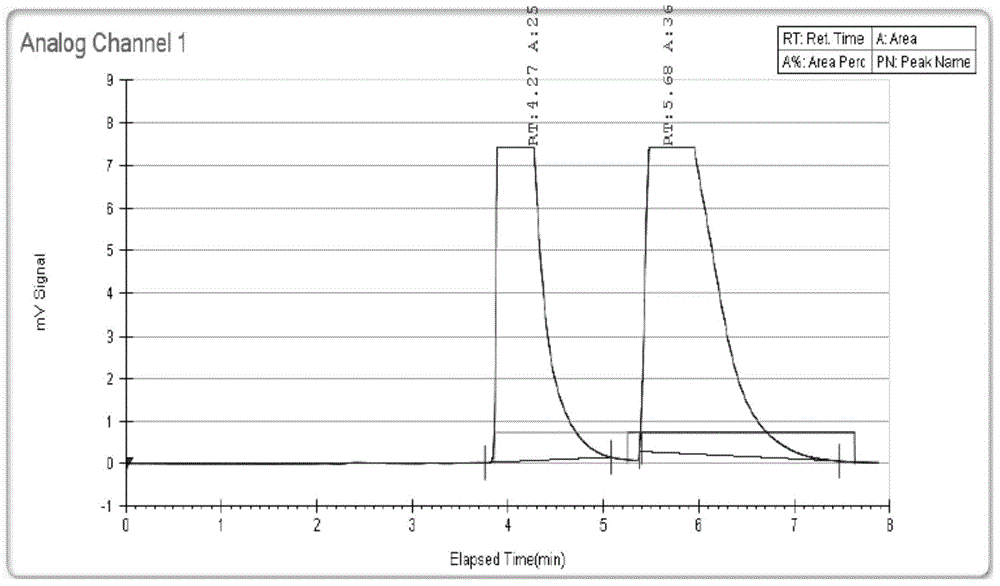
[0049] 1. Preparative chromatographic conditions are as follows:
[0050] Instrument: SFC350
[0051] Chromatographic column: CHIRALPAK AS-3 50*250mm, 50um.
[0052] Mobile phase: mobile phase A: 0.1% acetic acid methanol solution, mobile phase B: liquid carbon dioxide;
[0053] Mobile phase gradient program: mobile phase A 50%, mobile phase B 50%,
[0054] Detection wavelength: 220nm;
[0055] Column temperature: 34;
[0056] Injection volume: 4mL;
[0057] Flow rate: 160mL / min;
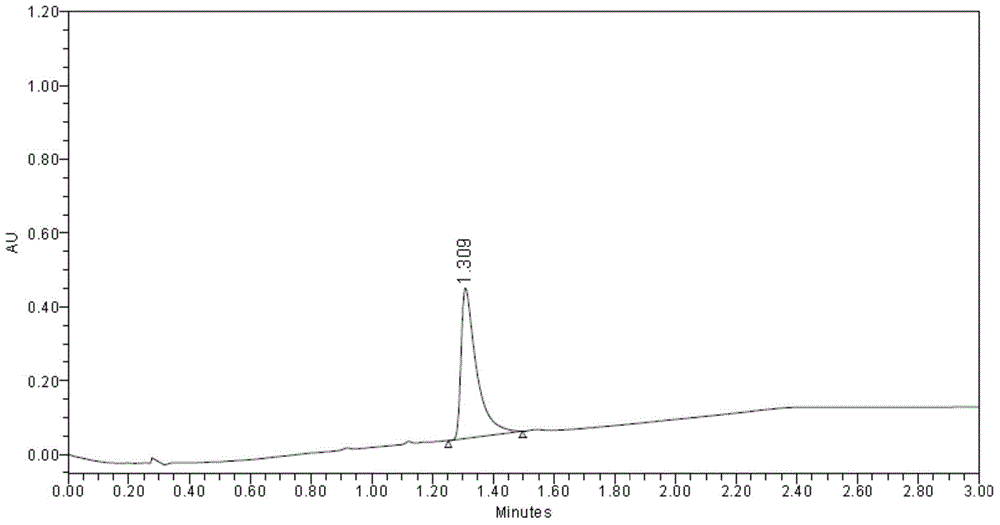
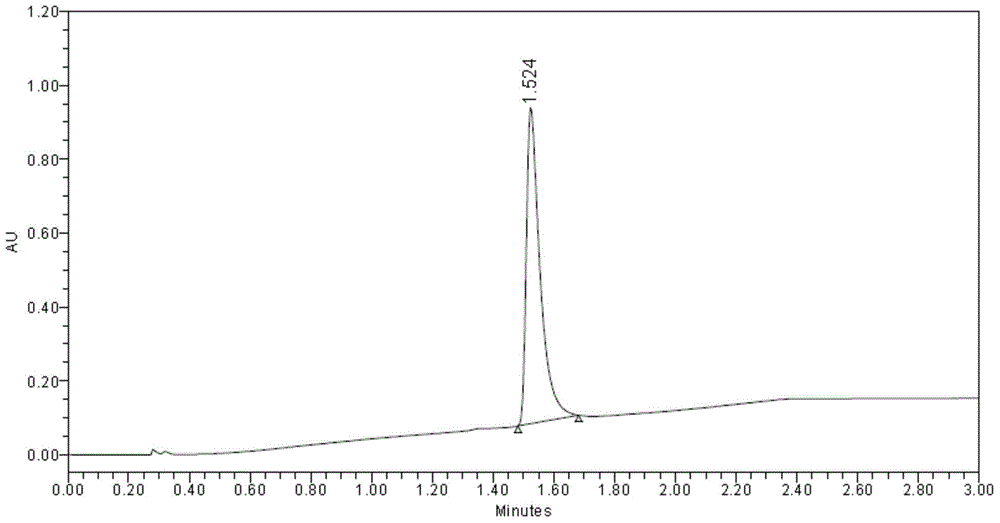
[0058] The corresponding component peaks were collected, and the collection time of the front peak was from 3.761 minutes to 5.074 minutes, which was identified as L-(-)-2-(5-bromo-4-(4-cyclopropylnaphthalene-1-yl)-4H-1 ,2,4-triazol-3-ylthio)acetic acid (the specific rotation is [α] 20 D =—9.5゜~—12.5゜, C=1, CH 3 OH); after peak collection t...
Embodiment 3
[0070] Example 3: Preparation of optically pure 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid sodium salt
[0071] Aqueous sodium hydroxide solution (1M, 2.0 mL, 2.0 mmol) was added dropwise to (-) or (+)-2-(5-bromo-4-(4-cyclopropylnaphthalene) at 10 °C over 5 minutes In a solution of -1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid (810 mg, 2.0 mmol) in ethanol (10 mL). The mixture was stirred for a further 10 minutes at 10°C. The volatile solvent was removed under vacuum at 30°C, the residue was dissolved in 15 mL of redistilled water, and freeze-dried to obtain solid (-) or (+)-2-(5-bromo-4-(4-cyclopropylnaphthalene-1- yl)-4H-1,2,4-triazol-3-ylthio)sodium acetate (850mg), Ms (Elpos): m / z=404.28[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com