A heat-resistant freeze-drying protectant for swine fever live vaccine, preparation method and application thereof
A live swine fever vaccine, heat-resistant freeze-drying technology, applied in the field of veterinary biological products manufacturing, can solve problems such as side effects, achieve the effects of increasing stability, efficiently protecting antigen activity, and avoiding stress reactions and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
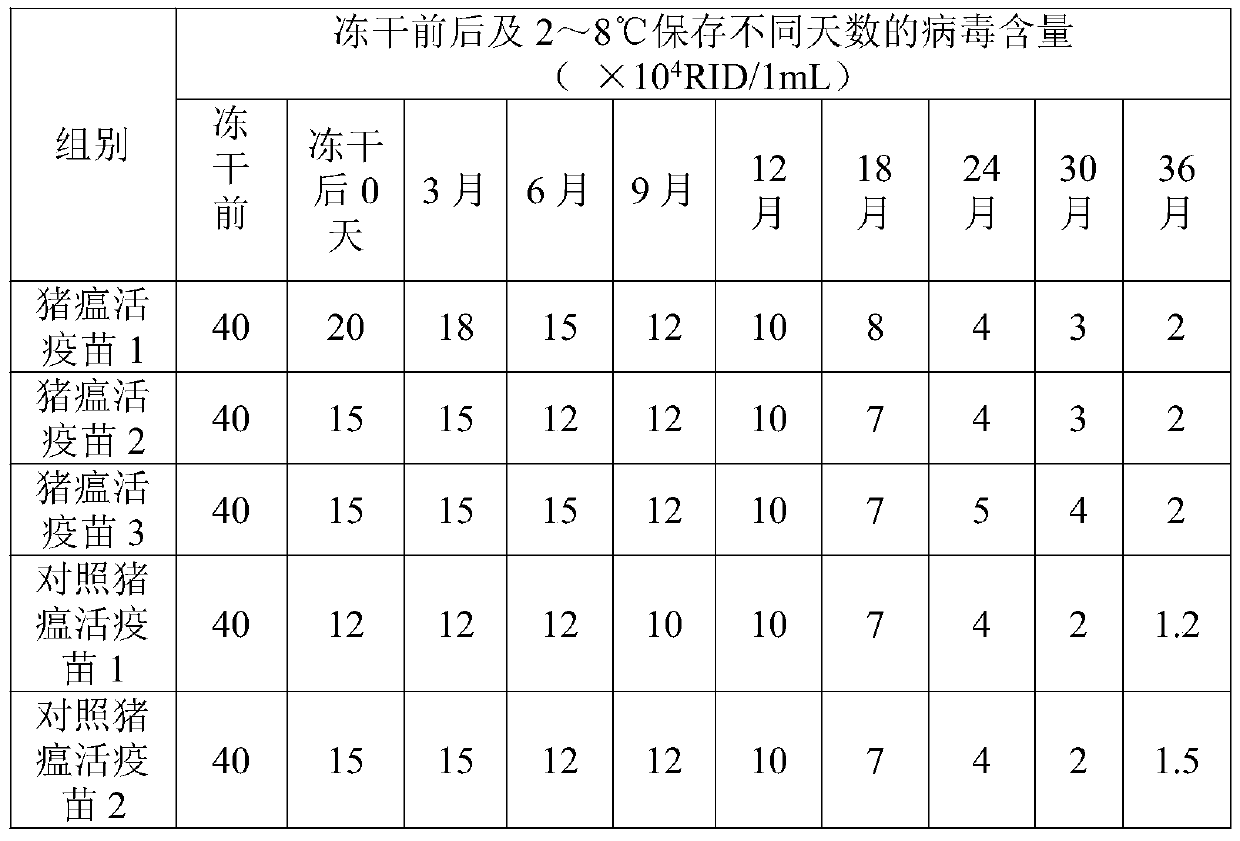
Image
Examples
Embodiment 1
[0021] Embodiment 1 heat-resistant lyoprotectant 1
[0022] The heat-resistant lyoprotectant 1 is composed of the following components in mass percentage: 0.5% inulin (polymerization degree (DP) is 9), 5% dextran (Dextran-70), polyvinylpyrrolidone (PVP K30 ) 3%, lactose 5%, sucrose 2%, polyethylene glycol (PEG 200) 1%, histidine 0.3%, cysteine 1.5%, DMEM medium (Gibco company) 3%, the rest is water for injection .
[0023] The specific preparation steps of the heat-resistant lyoprotectant 1 are as follows:
[0024] (1) Weigh inulin (DP is 9), dextran (Dextran-70), polyvinylpyrrolidone (PVP K30), lactose, sucrose, polyethylene glycol (PEG200) and dissolve in a small amount of water for injection, and steam Sterilize for 30 minutes, control the temperature at 110°C during the sterilization process, and set aside;
[0025] (2) Weigh histidine, cysteine and DMEM culture medium and dissolve in a small amount of water for injection, and use a filter membrane with a pore size ...
Embodiment 2
[0029] Embodiment 2 heat-resistant lyoprotectant 2
[0030] The heat-resistant lyoprotectant 2 is composed of the following components in mass percentage: inulin (DP 5) 2%, dextran (Dextran-70) 10%, polyvinylpyrrolidone (PVP K90) 2%, Lactose 1%, sucrose 0.5%, polyethylene glycol (PEG400) 2%, histidine 0.01%, cysteine 3%, DMEM medium 0.5%, and the rest is water for injection.
[0031] The specific preparation steps of the heat-resistant lyoprotectant 2 are as follows:
[0032] (1) Weigh inulin (DP 5), dextran (Dextran-70), polyvinylpyrrolidone (PVP K90), lactose, sucrose, polyethylene glycol (PEG400) and dissolve them in a small amount of water for injection. Sterilize for 30 minutes, control the temperature at 110°C during the sterilization process, and set aside;
[0033] (2) Weigh histidine, cysteine and DMEM and dissolve them in a small amount of water for injection, filter and sterilize them with a filter membrane with a pore size of 0.22 μm for later use;
[0034] ...
Embodiment 3
[0037] Embodiment 3 swine fever novel heat-resistant lyoprotectant 3
[0038]Novel heat-resistant lyoprotectant 3 for swine fever, consisting of the following components in mass percentage: inulin (DP 2) 0.2%, dextran (Dextran-40) 1%, polyvinylpyrrolidone (PVP K30) 5%, lactose 10%, sucrose 5%, polyethylene glycol (PEG 250) 0.1%, histidine 0.5%, cysteine 0.1%, DMEM medium 1.5%, and the rest is water for injection.
[0039] The specific preparation steps of the heat-resistant lyoprotectant 3 are as follows:
[0040] (1) Weigh inulin, dextran (Dextran-40), polyvinylpyrrolidone (PVP K30), lactose, sucrose, and polyethylene glycol, dissolve them in a small amount of water for injection, and sterilize them by autoclaving for 30 minutes. During the sterilization process, the temperature is controlled at 110°C for standby;
[0041] (2) Weigh histidine, cysteine and DMEM and dissolve them in a small amount of water for injection, filter and sterilize them with a filter membrane w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com