Method for synthesizing 5-aminomethyl-2-furfuryl alcohol
A technology of furan methanol and a synthesis method, applied in directions such as organic chemistry, can solve the problems of large amount of ammonia, high cost, low product yield and the like, and achieve the effects of less ammonia amount, mild conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
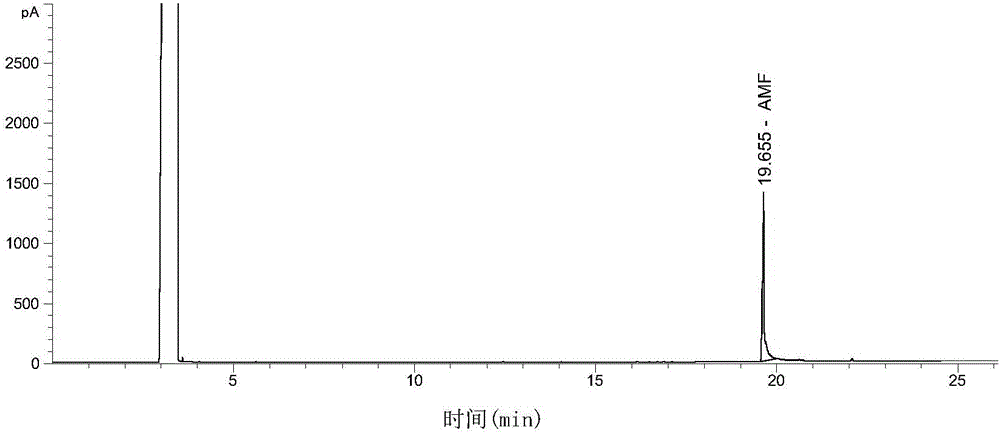
[0017] Add 10.0g of 5-hydroxymethylfurfural, 40mL of ammonia water with a concentration of 25% to 28%, 150mL of deionized water, 0.5g of Raney nickel in a 400mL autoclave, and feed in 1.2MPa of hydrogen to react at 80°C The reaction was stirred for 5h. After the reaction, the catalyst is recovered by filtration, the filtrate is transferred to a distillation flask, water and ammonia are removed by atmospheric distillation, and the residue is distilled under reduced pressure at 102-105°C and 0.1 Torr to obtain 5-aminomethyl-2-furanmethanol product (see figure 1 ), the product distillation yield exceeds 72%.
Embodiment 2
[0019] In a 400mL autoclave, add 10.0g of 5-hydroxymethylfurfural, 50mL of ammonia water with a concentration of 25% to 28%, 20mL of deionized water, 150mL of methanol, 0.5g of Raney nickel, and feed 1.0MPa of hydrogen into the The reaction was stirred at 100°C for 4h. After the reaction is finished, filter and recover the catalyst, transfer the filtrate to a distillation flask, and distill under normal pressure to recover the solvent and remove ammonia, and distill the residue under reduced pressure at 102-105°C and 0.1 Torr to obtain 5-aminomethyl-2-furan Methanol product, the product distillation yield exceeds 79%.
Embodiment 3
[0021] In a 400mL autoclave, add 10.0g of 5-hydroxymethylfurfural, 20mL of ammonia water with a concentration of 25% to 28%, 10mL of deionized water, 180mL of methanol, 0.5g of Raney nickel, and feed 1.2MPa of hydrogen into the The reaction was stirred at 100°C for 4h. After the reaction is finished, filter and recover the catalyst, transfer the filtrate to a distillation flask, and distill under normal pressure to recover the solvent and remove ammonia, and distill the residue under reduced pressure at 102-105°C and 0.1 Torr to obtain 5-aminomethyl-2-furan For methanol products, the product detection rate exceeds 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com