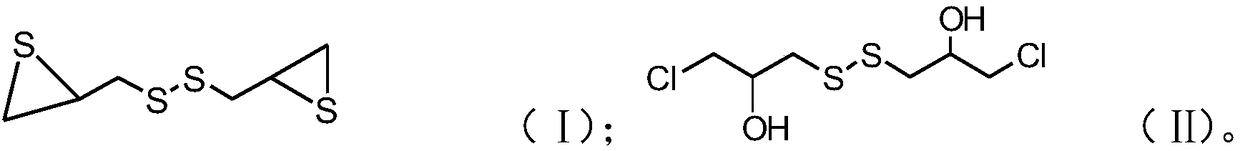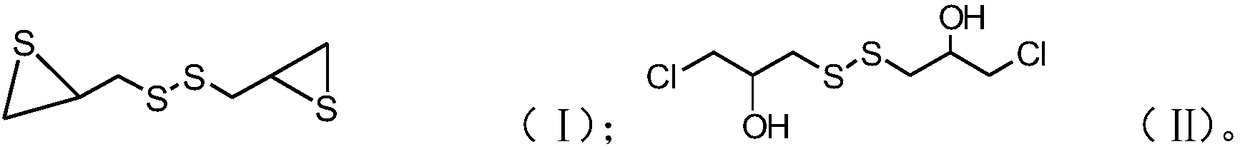The preparation method of episulfide compound
An episulfide compound and compound technology, applied in the field of preparation of episulfide compounds, can solve the problems of difficult and high yield episulfide compounds, slow reaction, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The embodiment of the present invention discloses a preparation method of an episulfide compound, comprising the following steps:
[0031] A), mixing one of epihalohydrin and brominated propylene oxide with a basic catalyst in a solvent to obtain a mixed solution;
[0032] B), mixing the mixed solution with the metal hydrosulfide solution, and reacting after passing through the air to obtain a compound with the structure of formula (II);
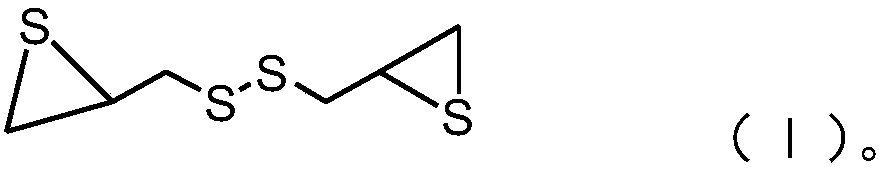
[0033] C), after mixing thiourea with a water-soluble solvent, add a catalyst, then add a compound with the structure of formula (II), cool after the first reaction, mix the obtained product with an alkaline solution, and obtain after the second reaction Episulfide compounds having the structure of formula (I);
[0034]
[0035] The present application provides a preparation method of episulfide compounds. According to the preparation method provided by the present invention, the reaction steps and the overall reaction time can be...
Embodiment 1
[0073] Weigh 1.9g of cobalt nitrate hexahydrate and 28.5g of molybdenum nitrate pentahydrate, and prepare them into aqueous solutions with a mass fraction of 30%, and mix them to obtain a nitrate mixed solution; then impregnate 100g of aluminum oxide in the above nitrate mixed solution, and Place it for 24 hours, then dry it at 150°C for 24 hours, and finally bake it in a muffle furnace at 500°C. After 4 hours, 10% Co 2 o 3 -MoO 2 / Al 2 o 3 catalyst.
Embodiment 2~3
[0075] According to the method of embodiment 1, adopt different carrier ZSM-5 and CeO 2 10% Co 2 o 3 -MoO 2 / ZSM-5 catalyst and 10% Co 2 o 3 -MoO 2 / CeO 2 catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com