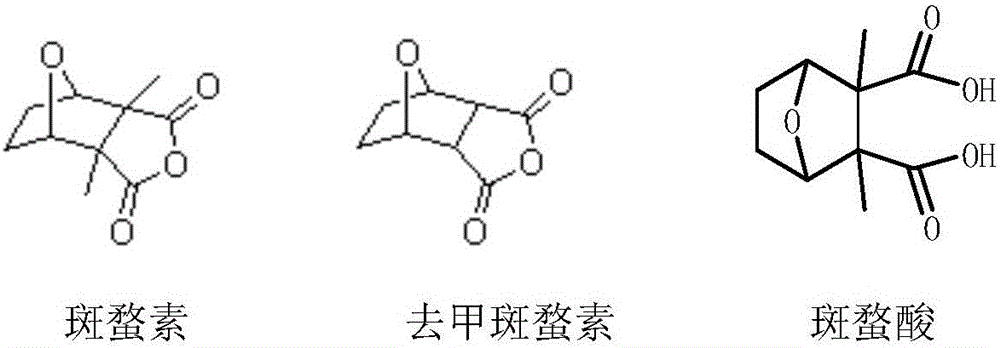
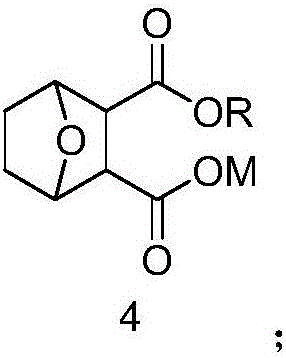
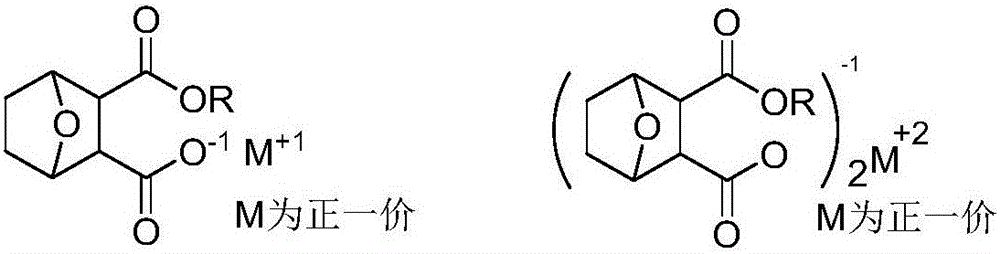
Norcantharidin monoester salt derivative and anti-tumor application thereof
A technology of norcantharidin and its derivatives, which is applied in the field of new norcantharidin monoester salt derivatives and their anti-tumor applications, and can solve the problems of low bioavailability, poor water solubility, and no reported norcantharidin monoester. The structure and synthesis method of ester salt derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0037] The preparation of embodiment 1.5-ene norcantharidin 1
[0038]Take out a certain amount of maleic anhydride from the reagent bottle, place it in a dry grinding body and grind it finely, then weigh 12.021g of the finely ground maleic anhydride with an electronic balance, put it in a dry three-necked flask, and plug it Stopper, add diethyl ether and stir, when the amount of diethyl ether is 90mL, the maleic anhydride is completely dissolved. After the maleic anhydride was completely dissolved, 13 mL of furan was slowly added through the dropping funnel for 13 minutes. The temperature was controlled to start the reaction at 38°C. After reacting for 1 h, white solids appeared in the solution, and the longer the time, the more white solids there were. After reacting for 24 hours, it was filtered with suction to obtain compound 1 as a white solid, that is, 5-enorcantharidin. The dry weight was 17.46g, and the yield was 85.8%. Melting point: 122-123°C, ratio shift Rf: 0.5...
Embodiment 2
[0039] Embodiment 2. The preparation of norcantharidin 2
[0040] Take 5-enenorcantharidin 1 (1.09g) in a Schlenk bottle, add 20ml of tetrahydrofuran to dissolve it, add 110mg of palladium carbon, remove the air in the flask by vacuum, then feed hydrogen, stir at 25°C to make it react, After the reaction was finished, palladium carbon was removed by suction filtration, and the resulting filtrate was rotary evaporated and dried to obtain norcantharidin (72.2%), 794.3 mg of white solid. 1H NMR (DMSO-d6): δ: 4.85 (s, 2H), 3.34 (d, J=20Hz, 2H), 1.65 (d, J=8Hz, 4H). 13CNMR (DMSO-d6): δ: 173.35, 80.08, 51.14, 40.35, 40.14, 39.93, 39.72, 27.90.
Embodiment 3
[0041] Embodiment 3. the synthesis of norcantharidin monoethyl ester (3, R=Et):
[0042] Take 672mg of norcantharidin 2 in a flask, add 30ml of absolute ethanol to dissolve it, heat to reflux at 80°C, after 4.5 hours, the reaction is complete, rotary steam, use ethyl acetate as the eluent, pass through a silica gel column, spot the plate, and collect The colored sample was rotary evaporated and dried to obtain norcantharidin monoacid ethyl ester (87%), 747.2 mg of white solid. 1HNMR (CDCl3): δ: 4.91(d, J=24Hz, 2H), 4.11(d, J=8Hz, 2H), 2.99(q, J=12Hz, 3H), 1.81(t, J=4Hz, 2H) , 1.52 (d, J=8Hz, 2H), 1.21 (t, J=8Hz, 3H). 13CNMR (CDCl3): δ: 176.45, 170.87, 78.59, 78.29, 77.03, 76.71, 61.16, 52.27, 28.97, 13.94.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com