Single-chain antibody of swine-origin enterotoxigenic Escherichia coli K88 FaeG resistant protein and preparation method of single-chain antibody
A single-chain antibody and Escherichia coli technology, applied in the field of genetic engineering, can solve problems such as the unsatisfactory effect of antibiotics on bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
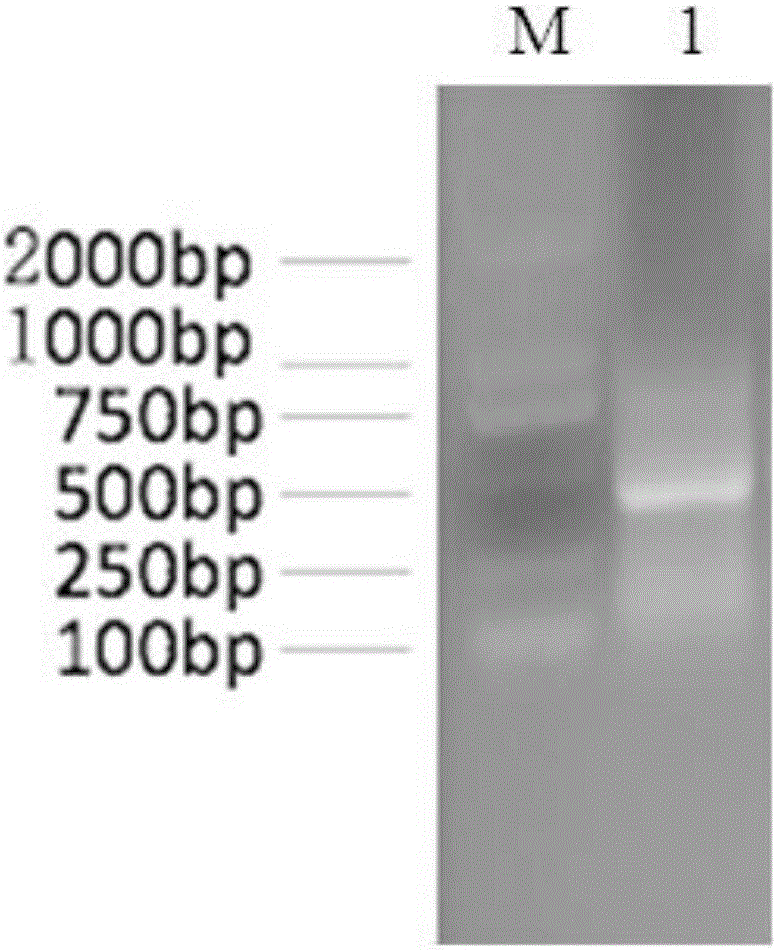
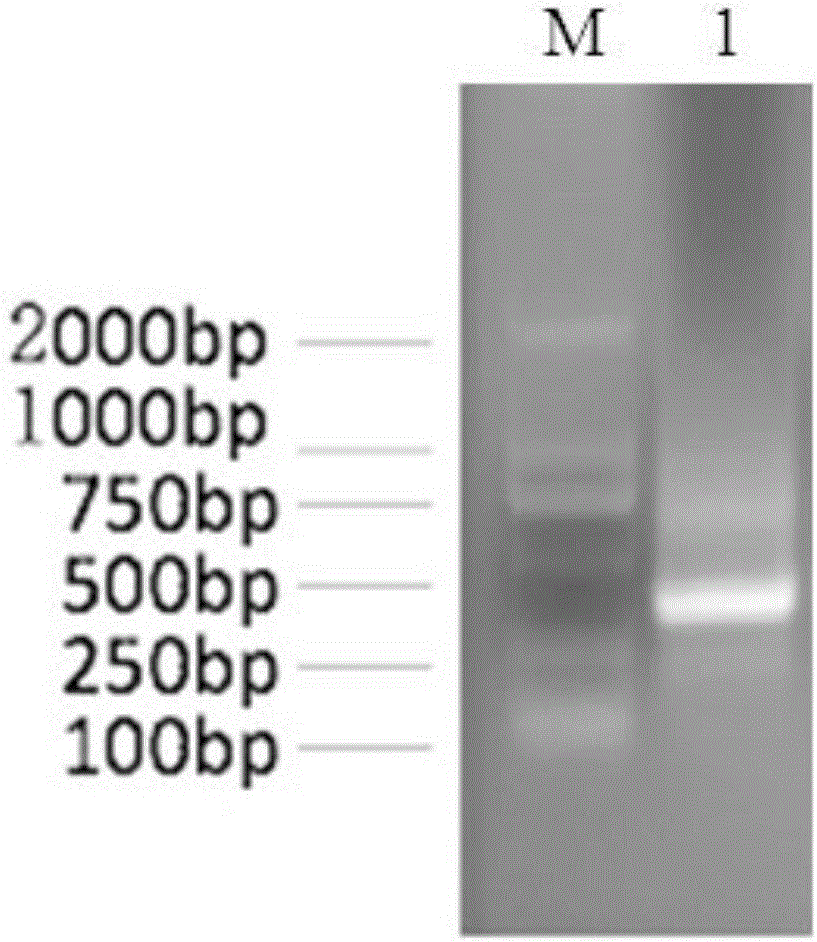
[0027] Example 1 Preparation of the single-chain antibody of pig-derived anti-enterotoxigenic Escherichia coli K88FaeG protein
[0028] 1. The heavy chain variable region sequence (AF064686.1; AF064687.1; AF064688.1; AF064689.1; AF064690.1) and light chain variable region sequence (KF561242.1) of the porcine antibody coding gene published by GenBank; GQ867594.1; GQ867595.1) designed primers for amplifying antibody light and heavy chains (Table 1), in which VH1F, VH1R and VH2R were used to amplify the VH region; VL1F, VL2F, VL3F and VL1R were used to amplify the VL region ; VH3F and VH3R are used to add restriction sites and Linker sequences to VH genes; VL4F, VL5F and VL6F are used to add restriction sites to VL genes amplified by VL1F, VL2F and VL3F, and VL2R is used to add Linker sequences to VL genes. Among them, VH3F contains a Sfi I restriction site, VL 2R contains a Not I restriction site; VH3R, VL4F, VL5F, and VL6F contain complementary Linker sequences (the restriction...
Embodiment 2
[0043] Example 2 Indirect ELISA screening of antigen-specific ScFv
[0044] Take the purified prokaryotically expressed ETEC K88FaeG protein (prepared in our laboratory), dilute it to 5 μg / mL with antigen coating solution (50 mmol / L sodium bicarbonate solution, pH=9.6), add it to a 96-well plate, 100 μL per well, Coat overnight at 4°C; add 200 μL of 5% skimmed milk powder solution to each well, block at 37°C for 1 hour, and wash 3 times with PBS; mix 50 μL of purified protein supernatant with 50 μL of 4% skim milk powder solution and add to the above wells, at 37°C Incubate for 2 h, wash with PBST 3 times; add 100 μL (1:2000) of E-Tag Mouse mAb (E-tag mouse monoclonal antibody purchased from RayBiotech Company), react at 37°C for 2 h, wash with PBST 3 times; add hydrogen peroxide to label Goat anti-mouse IgG secondary antibody (purchased from Invitrogen, USA) 1000 μL (1:4000), reacted at 37°C for 1 h, washed 3 times with PBST; developed color with TMB for 15 min, terminated re...
Embodiment 3
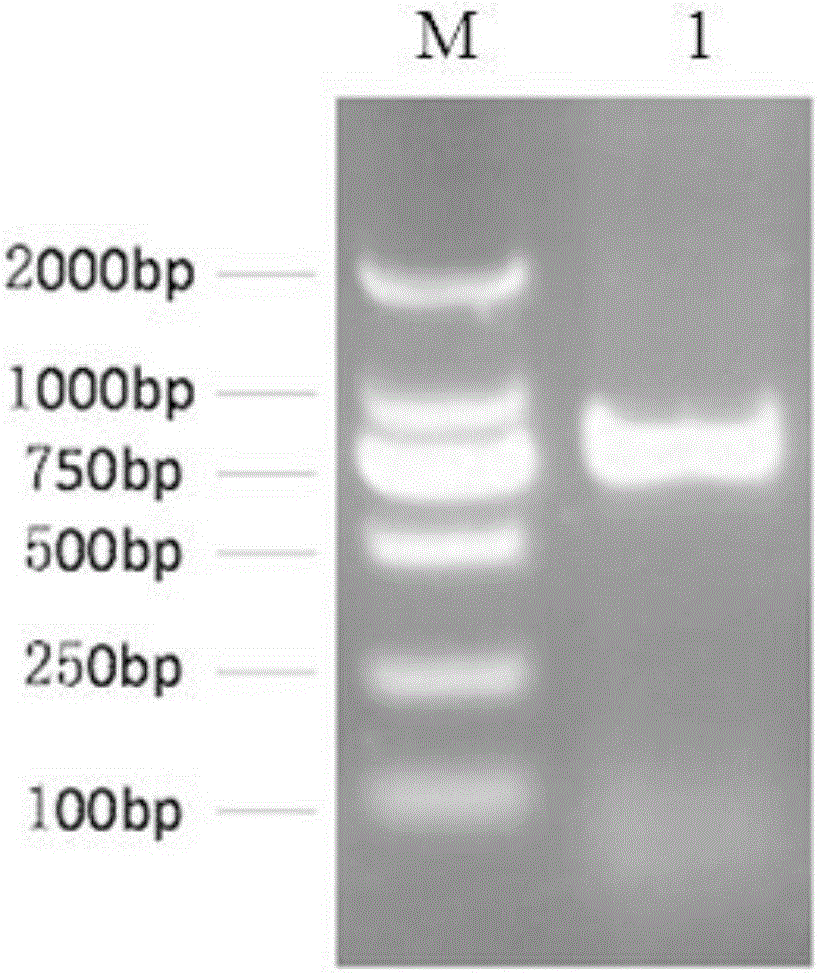
[0046] The obtained single-chain antibody coding gene was sequenced to prove that it consists of 762 nucleotides and 254 amino acids deduced accordingly. The nucleotide sequence is shown in SEQ ID No: 4, and the amino acid sequence is shown in SEQ ID No: 4. ID No: 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com