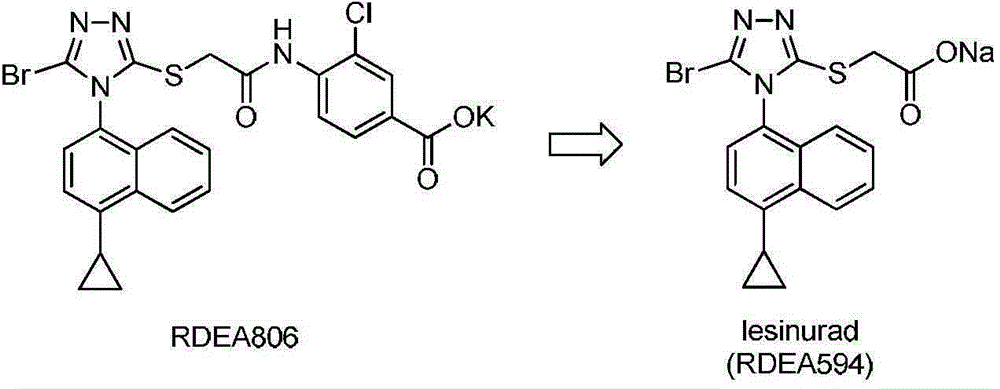
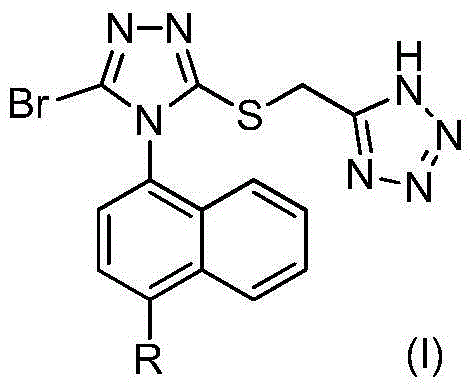
Tetrazole type URAT1 inhibitors, preparing method, and uses of the inhibitors in treatment of hyperuricemia and gout
An OR2, C1-C4 technology, applied in the field of uric acid transporter 1 inhibitors, can solve the problems of serious side effects, unsuitable long-term treatment, renal function damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 compound I-1
[0036]
[0037] Step 1. Synthesis of compound IV-1
[0038]Compound II-1 (2.82g, 10mmol), Compound III (1.78g, 15mmol), KI (1.66g, 10mmol) and K 2 CO 3 (4.15g, 30mmol) was added to a dry 250mL round bottom flask and DMF (100mL) was added. The resulting reaction mixture was stirred at 80° C. under a nitrogen atmosphere until TLC indicated that the reaction was complete (typically around 6 h).
[0039] After the reaction mixture was cooled, it was poured into ice water (1000mL), stirred, the pH of the resulting mixture was >7, and the mixture was washed with CH 2 Cl 2 (200mL×3) extraction, the organic phase was discarded, and the aqueous phase was adjusted to pH = 3 with concentrated hydrochloric acid, and again with CH 2 Cl 2 (300 mL×5) extraction. Combine and extract the organic phases, wash with 5% brine, anhydrous Na 2 SO 4 dry. The dried organic phase was evaporated to remove the solvent on a rotary evaporato...
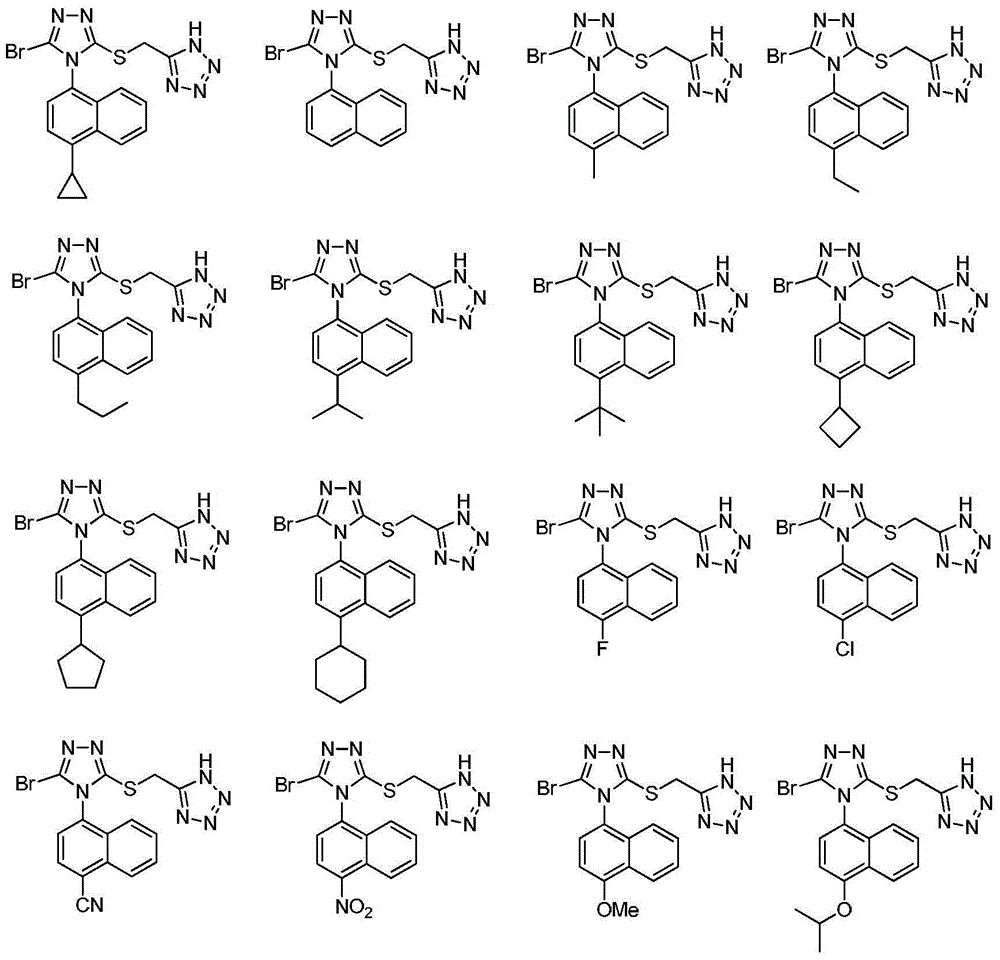
Embodiment 2-16
[0049] Synthesis of Example 2-16 Compounds I-2 to I-16
[0050] Referring to the method of Example 1, the following compounds with general formula I were synthesized.
[0051]
[0052]
[0053]
Embodiment 17
[0054] Example 17 Synthesis of sodium salt I-S-1 from compound I-1
[0055]
[0056] Compound I-1 (0.428g, 1mmol) was dissolved in methanol (5mL), stirred at room temperature, slowly added a solution prepared by NaOH (0.400g, 1mmol) and water (1mL), after the addition, the reaction mixture was at room temperature Stirring was continued for 10 minutes.
[0057] The reaction mixture was evaporated to dryness on a rotary evaporator, and the obtained residue was dissolved in methanol (20mL×2) and then evaporated to dryness to remove water in the residue, and the obtained residue was further dried in a water bath at 35°C on a vacuum oil pump After 12 hours, the sodium salt I-S-1 of I-1 was obtained as a white solid, 0.441 g, with a yield of 98%. 1 H NMR (DMSO-d 6 ,400MHz),δ8.55(d,1H,J=8.4Hz),7.71(t,1H,J=7.4Hz),7.62(t,1H,J=7.6Hz),7.56(d,1H,J= 7.6Hz), 7.40(d, 1H, J=7.6Hz), 7.12(d, 1H, J=8.4Hz), 4.47(d, 1H, J=14.0Hz), 4.43(d, 1H, J=14.4Hz ),2.51-2.56(m,1H),1.11-1.15(m,2H),0.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com